Answered step by step
Verified Expert Solution
Question
1 Approved Answer
4. A disaccharide X cannot be oxidised by bromine water. The acid hydrolysis of X leads to a laevorotatory solution. The disaccharide X is
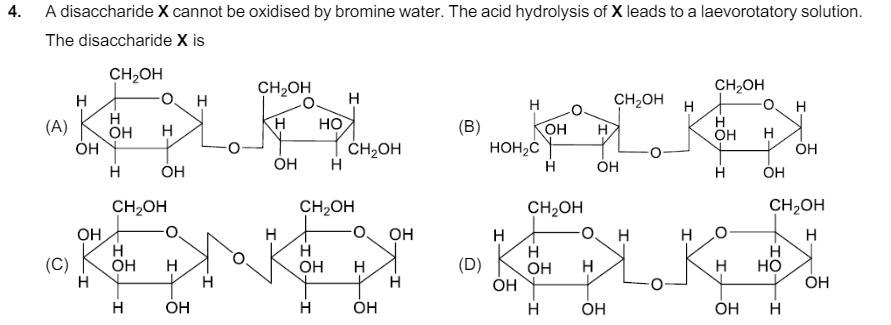


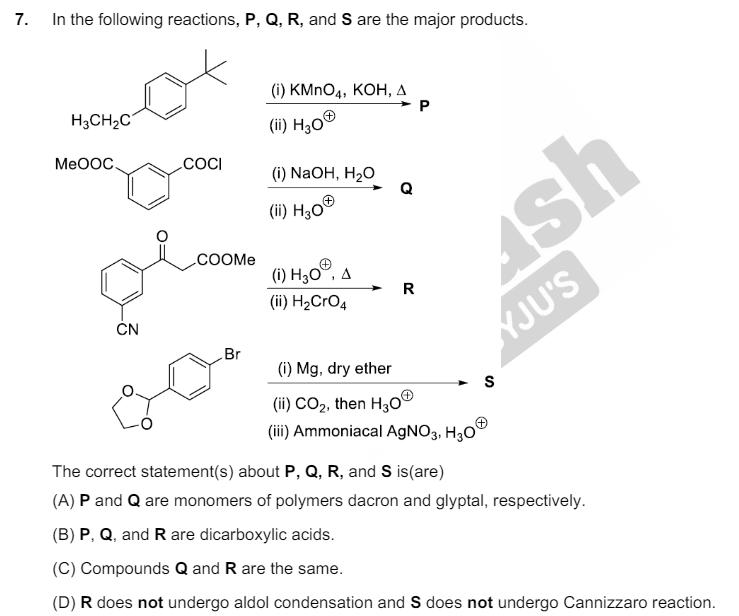
4. A disaccharide X cannot be oxidised by bromine water. The acid hydrolysis of X leads to a laevorotatory solution. The disaccharide X is (A) CHOH H H H CHOH O. CHOH H H CHOH H H H HO OH H (B) H HO H OH H OH CHOH HOHC OH H HO H H OH OH (C) OH H OH CHOH H OH H H OH H CH2OH H OH H OH H OH H H (D) CHOH H OH H HO I CHOH H H HO OH H HO H HO 5. The complex(es), which can exhibit the type of isomerism shown by [Pt(NH3)2Br2], is (are) [en = H2NCH2CH2NH2] (A) [Pt(en)(SCN)2] (B) [Zn(NH3)2Cl2] (C) [Pt(NH3)2C14] (D) [Cr(en)2(H2O)(SO4)]* 6. Atoms of metals x, y and z form face-centred cubic (fcc) unit cell of edge length Lx, body-centred cubic (bcc) unit cell of edge length Ly, and simple cubic unit cell of edge length Lz, respectively. 3 8 If r = ry; ry= 2 3 3 rx; M = My and M = 3Mx, then the correct statement(s) is (are) [Given: Mx, My, and Mz are molar masses of metals x, y, and z, respectively. rx, ry, and rz are atomic radii of metals x, y, and z, respectively.] (A) Packing efficiency of unit cell of x > Packing efficiency of unit cell of y > Packing efficiency of unit cell of z (B) Ly > Lz (C) Lx > Ly (D) Density of x > Density of y 7. In the following reactions, P, Q, R, and S are the major products. H3CH2C (i) KMnO4, KOH, A (ii) H3O P MeOOC. COCI (i) NaOH, HO (ii) H3O COOME (i) H3O, A R (ii) H2CrO4 CN Br 4sr YJU'S (i) Mg, dry ether (ii) CO2, then H3O (iii) Ammoniacal AgNO3, H3O The correct statement(s) about P, Q, R, and S is(are) S (A) P and Q are monomers of polymers dacron and glyptal, respectively. (B) P, Q, and R are dicarboxylic acids. (C) Compounds Q and R are the same. (D) R does not undergo aldol condensation and S does not undergo Cannizzaro reaction.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


