Question: 4. Ascorbic acid is going to be determined by putting a known excess of iodine formed by the action of 10, on KI and
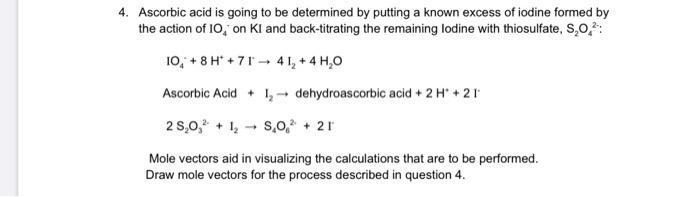
4. Ascorbic acid is going to be determined by putting a known excess of iodine formed by the action of 10, on KI and back-titrating the remaining lodine with thiosulfate, S,0,: 10, + 8 H* + 71 - 4 1, + 4 H,0 Ascorbic Acid + 1- dehydroascorbic acid + 2 H' + 21 2 S,0, + 1, - S,o, + 21 Mole vectors aid in visualizing the calculations that are to be performed. Draw mole vectors for the process described in question 4.
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


