Question
4. Ethylene oxide (C2H4O) is produced by the reaction of ethylene (C2H4) with oxygen: 2C2H4+ O22C2H4O The feed to the reactor contains 5 mol/h
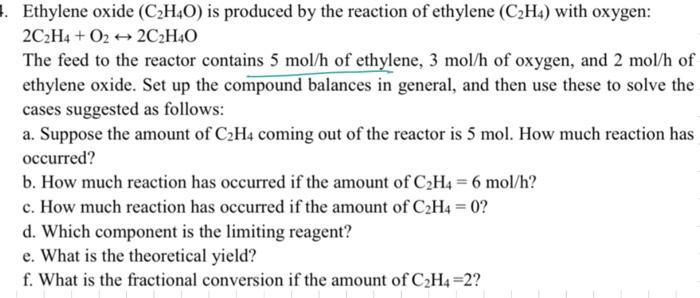
4. Ethylene oxide (C2H4O) is produced by the reaction of ethylene (C2H4) with oxygen: 2C2H4+ O22C2H4O The feed to the reactor contains 5 mol/h of ethylene, 3 mol/h of oxygen, and 2 mol/h of ethylene oxide. Set up the compound balances in general, and then use these to solve the cases suggested as follows: a. Suppose the amount of C2H4 coming out of the reactor is 5 mol. How much reaction has occurred? b. How much reaction has occurred if the amount of C2H4-6 mol/h? c. How much reaction has occurred if the amount of C2H4=0? d. Which component is the limiting reagent? e. What is the theoretical yield? f. What is the fractional conversion if the amount of C2H4=2?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
College Algebra With Modeling And Visualization
Authors: Gary Rockswold
6th Edition
0134418042, 978-0134418049
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



