Answered step by step
Verified Expert Solution
Question
1 Approved Answer
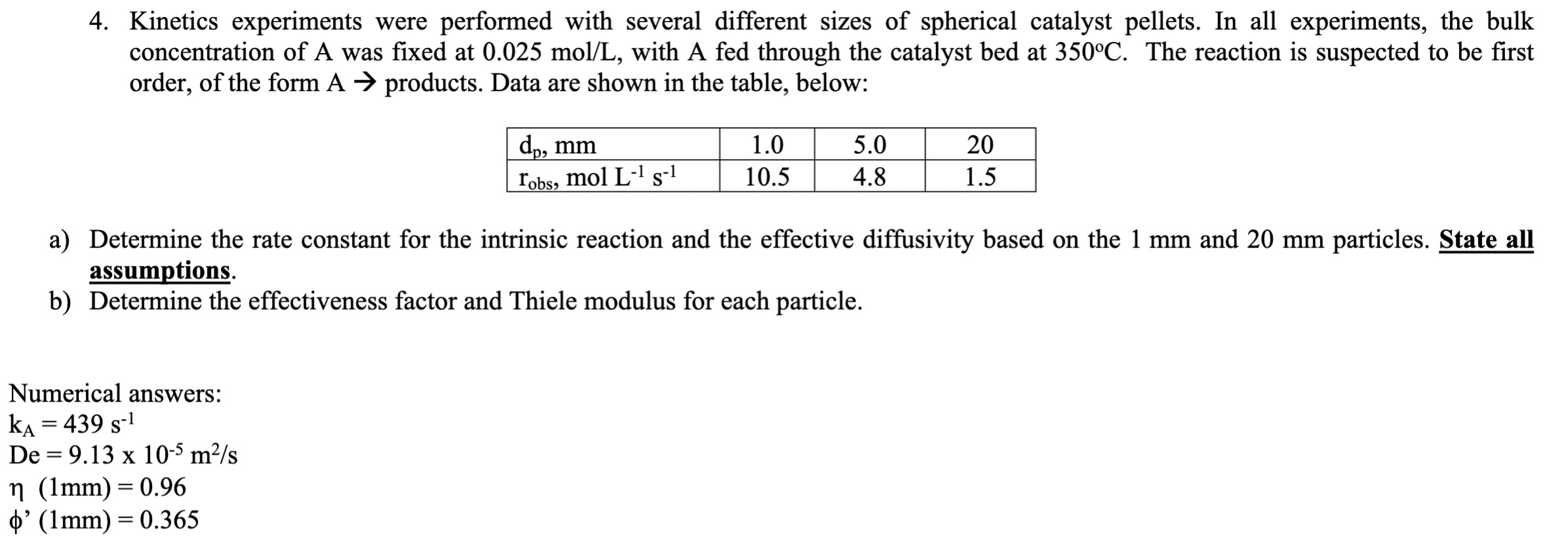
4 . Kinetics experiments were performed with several different sizes of spherical catalyst pellets. In all experiments, the bulk concentration of A was fixed at
Kinetics experiments were performed with several different sizes of spherical catalyst pellets. In all experiments, the bulk concentration of A was fixed at molL with A fed through the catalyst bed at deg C The reaction is suspected to be first order, of the form A products. Data are shown in the table, below:dommJobs, mol La Determine the rate constant for the intrinsic reaction and the effective diffusivity based on the mm and mm particles. State all assumptions.b Determine the effectiveness factor and Thiele modulus for each particle.Numerical answers: kxDe x msn mmmm
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


