Answered step by step
Verified Expert Solution
Question
1 Approved Answer
4. Mono-Methyl Hydrazine (CH6N2) is to be used as a fuel, with Nitrogen Tetroxide (N2O4) as an oxidizer, in a combustor, a. What is
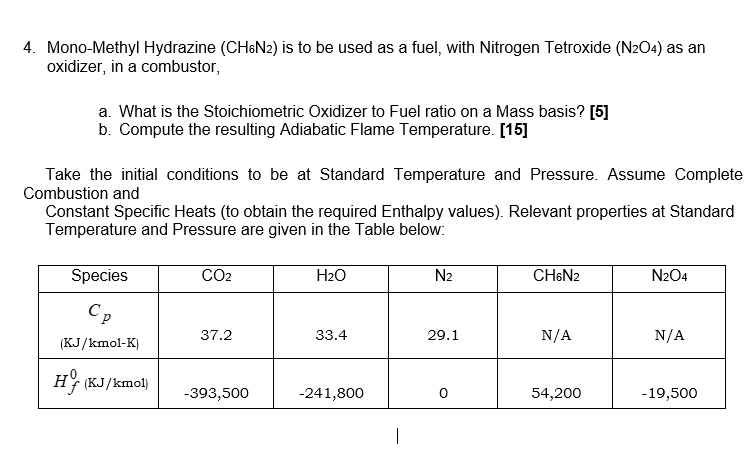
4. Mono-Methyl Hydrazine (CH6N2) is to be used as a fuel, with Nitrogen Tetroxide (N2O4) as an oxidizer, in a combustor, a. What is the Stoichiometric Oxidizer to Fuel ratio on a Mass basis? [5] b. Compute the resulting Adiabatic Flame Temperature. [15] Take the initial conditions to be at Standard Temperature and Pressure. Assume Complete Combustion and Constant Specific Heats (to obtain the required Enthalpy values). Relevant properties at Standard Temperature and Pressure are given in the Table below: Species Cp CO2 H2O N2 CH6N2 N2O4 37.2 33.4 29.1 N/A N/A (KJ/kmol-K) H (KJ/kmol) -393,500 -241,800 0 54,200 -19,500 |
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


