Answered step by step
Verified Expert Solution
Question
1 Approved Answer
(4 points) When you get your oil changed (assume light oil), the mechanic adds your dirty and hot 4 quarts of oil to the recycling
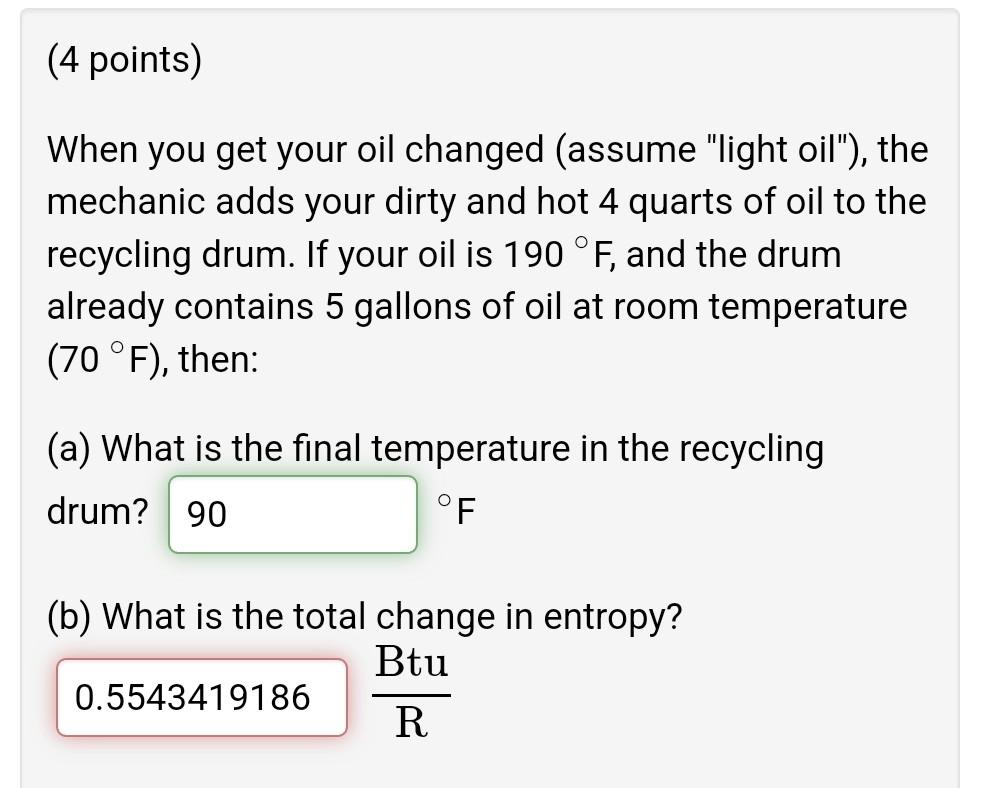
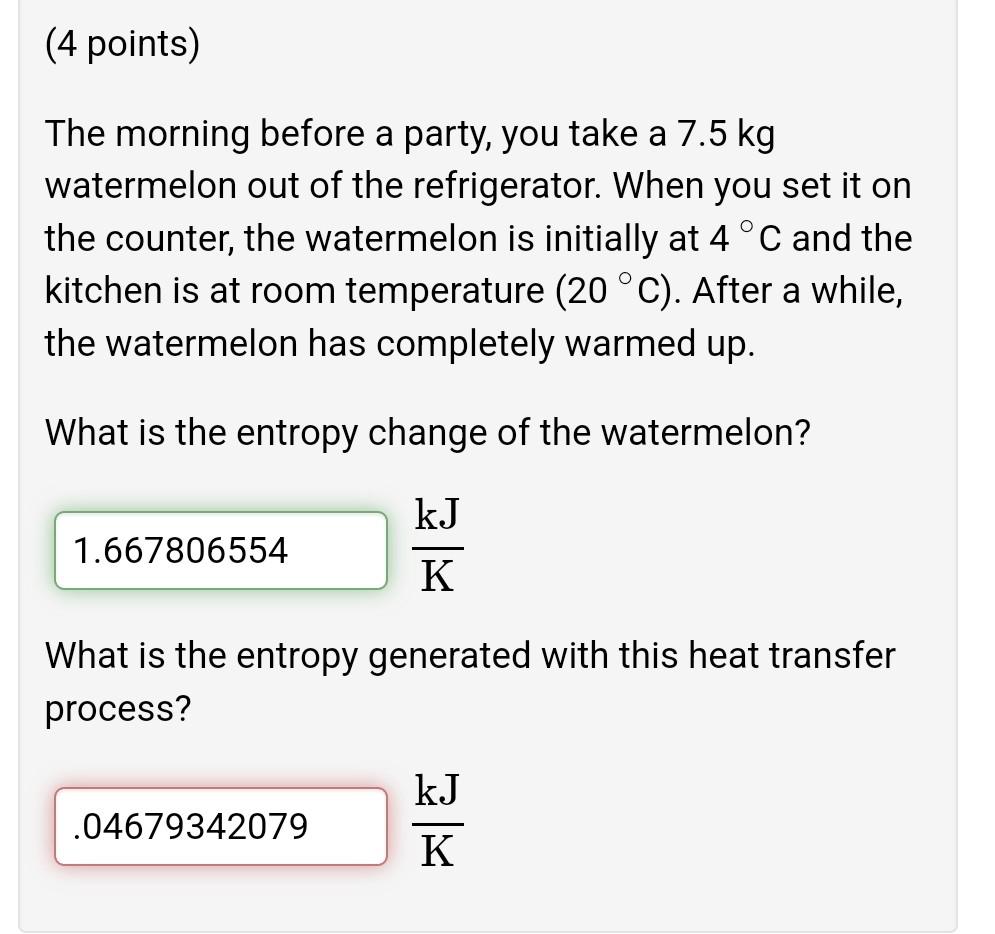
(4 points) When you get your oil changed (assume "light oil"), the mechanic adds your dirty and hot 4 quarts of oil to the recycling drum. If your oil is 190 F, and the drum already contains 5 gallons of oil at room temperature (70 F), then: (a) What is the final temperature in the recycling drum? 90 F (b) What is the total change in entropy? Btu 0.5543419186 R (4 points) The morning before a party, you take a 7.5 kg watermelon out of the refrigerator. When you set it on the counter, the watermelon is initially at 4 C and the kitchen is at room temperature (20 C). After a while, the watermelon has completely warmed up. What is the entropy change of the watermelon? kJ 1.667806554 K What is the entropy generated with this heat transfer process? kJ .04679342079 K
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


