Answered step by step
Verified Expert Solution
Question
1 Approved Answer
4. Reaction intermediates are neither a reactant nor a product of a reaction. They are chemical species formed during a reaction but later consumed before
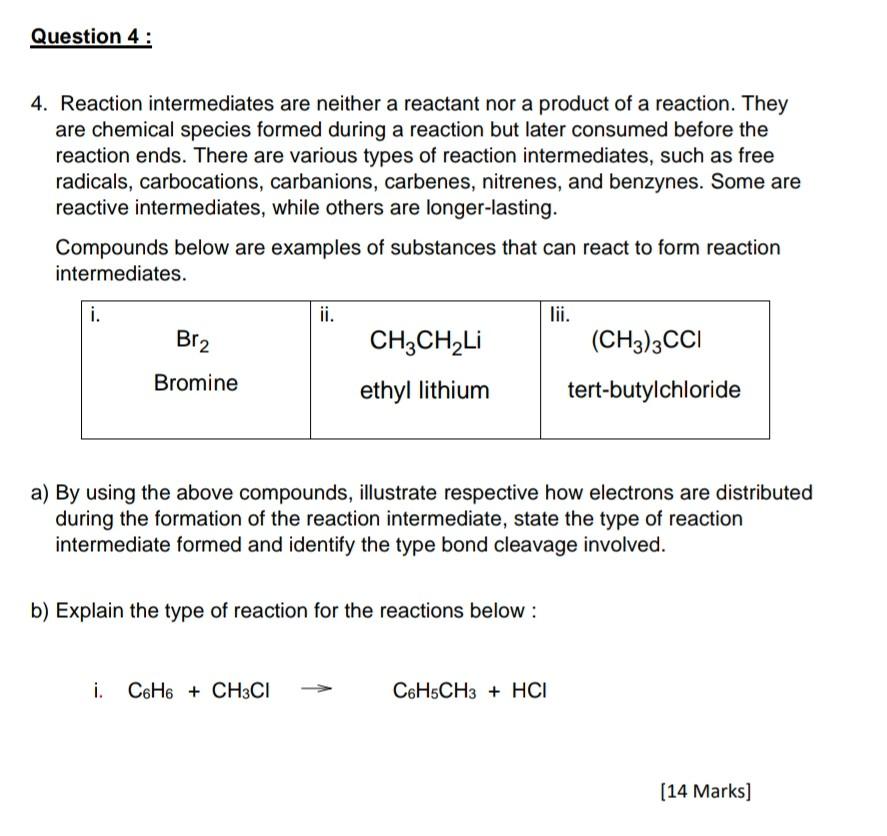
4. Reaction intermediates are neither a reactant nor a product of a reaction. They are chemical species formed during a reaction but later consumed before the reaction ends. There are various types of reaction intermediates, such as free radicals, carbocations, carbanions, carbenes, nitrenes, and benzynes. Some are reactive intermediates, while others are longer-lasting. Compounds below are examples of substances that can react to form reaction intermediates. a) By using the above compounds, illustrate respective how electrons are distributed during the formation of the reaction intermediate, state the type of reaction intermediate formed and identify the type bond cleavage involved. b) Explain the type of reaction for the reactions below : i. C6H6+CH3ClC6H5CH3+HCl
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


