Question
Pentaborane BH,(s) burns vigorously in O2 to give B2O3(s) and H2O(l). Calculate the heat given off, in KJ, for the combustion of 148.82 g
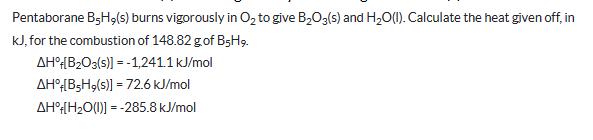
Pentaborane BH,(s) burns vigorously in O2 to give B2O3(s) and H2O(l). Calculate the heat given off, in KJ, for the combustion of 148.82 g of B5H9. AH[B2O3(s)]=-1,241.1 kJ/mol AH [BSH,(s)] 72.6 kJ/mol AH [H2O(1)]=-285.8 kJ/mol
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Business Statistics
Authors: Norean Sharpe, Richard Veaux, Paul Velleman
3rd Edition
978-0321944726, 321925831, 9780321944696, 321944720, 321944690, 978-0321925831
Students also viewed these Marketing questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



