Answered step by step
Verified Expert Solution
Question
1 Approved Answer
4. Your final project involved thermal methane decomposition into solid carbon and hydrogen gas. In reality, intermediate hydrocarbon species will form at relatively lower

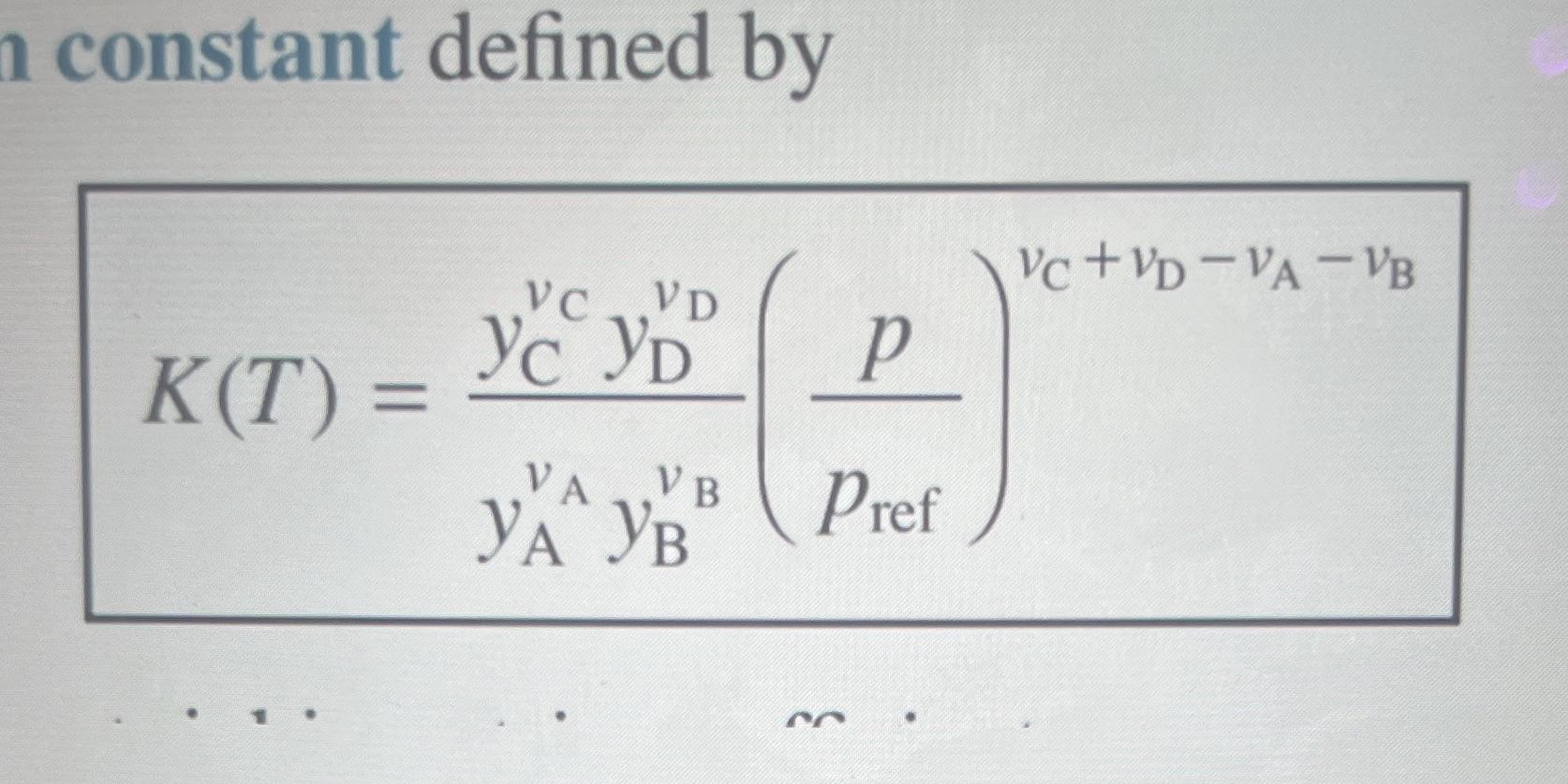

4. Your final project involved thermal methane decomposition into solid carbon and hydrogen gas. In reality, intermediate hydrocarbon species will form at relatively lower temperatures. Here, we revisit methane pyrolysis, but this time we assume an intermediate reaction with no solid carbon such that the equilibrium reaction takes the following form: 2CH4 C2H2(g) + 3H2(g) Methane enters the reaction chamber at 0.03 atm and 298 K. The products exit the reaction chamber as an equilibrium mixture of CH4, C2H2, and H2 at 0.03 atm and 1500 K. (Use the formal problem-solving template with the Known, Schematic & Given Data, and Find sections, and place your final answers in the boxes next to the corresponding part of the question). a) Write a system of equations that can be used to solve the composition of the products at 1500 K. Write this system of equations such that the equilibrium constant K (see "K-equation, Eq. 14.32 in the textbook) becomes a function of one unknown variable / concentration only. After making any necessary substitutions no simplification is required. All other equations should be given as expressions that can find the other concentrations as a function of this unknown variable that is present in the "K-equation." For example if a, b, and c are concentrations: K = f(a) b = f(a) c = f(a) n constant defined by VC+VD-VA-VB VD p V B Pref K(T) = VC Yc YD VA YA YB b) Solve the system of equations above to find the equilibrium concentrations of all species at 0.03 atm and 1500 K. You may assume that K = 0.03, and that the reference pressure is 1 atm. c) Suppose that the pressure in the reactor is increased to 0.3 atm while all other conditions stay the same. Would the equilibrium composition of hydrogen at the reactor exit increase, decrease, or stay the same? d) Now suppose that the temperature at the reactor exit increases 2000 K such that K=0.154. All other conditions remain the same (the pressure is still 0.03 atm, and reaction species are the same). Would the equilibrium composition of hydrogen at the reactor exit increase, decrease, or stay the same?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


