Answered step by step
Verified Expert Solution
Question
1 Approved Answer
4.10. Using the coefficients to evaluate the vapor pressure for Benzene listed in Table B.2 of App. B, evaluate the latent heat of vaporization Hn
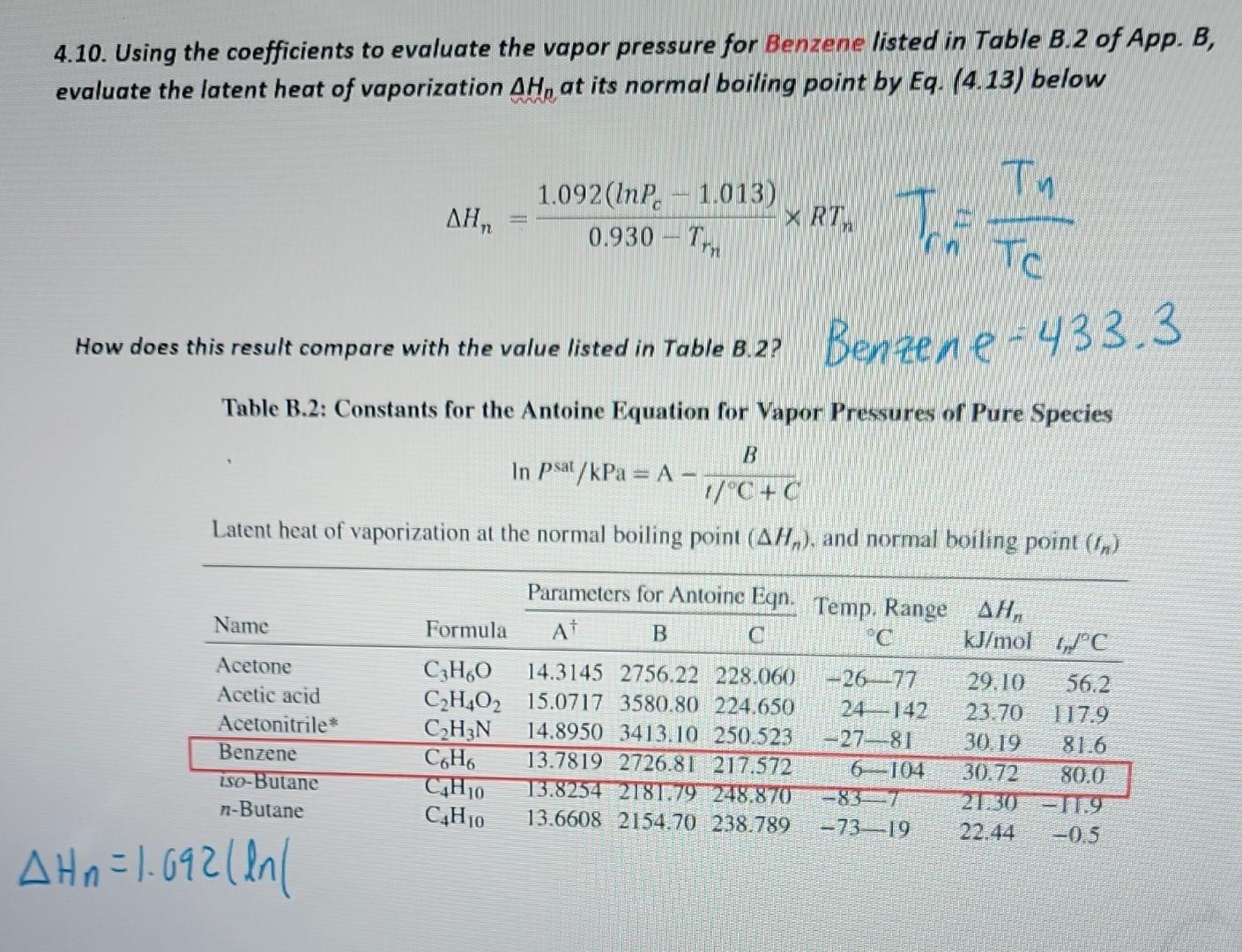
4.10. Using the coefficients to evaluate the vapor pressure for Benzene listed in Table B.2 of App. B, evaluate the latent heat of vaporization Hn at its normal boiling point by Eq. (4.13) below Hn=0.930Trn1.092(lnPc1.013)RTnTn=TCTn How does this result compare with the value listed in Table B.2? Ben Re, e=433.3 Table B.2: Constants for the Antoine Equation for Vapor Pressures of Pure Species lnPsat/kPa=A1/C+CB Latent heat of vaporization at the normal boiling point (Hn), and normal boiling point (tn) Hn=1.092(ln1
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


