Answered step by step
Verified Expert Solution
Question
1 Approved Answer
4,7 please answer all the questions ULY pxyiene 0.410 mol Moles of p-xylene 0.4104 mol Mass of water 20.09 b Calculate the change in heat
4,7 please answer all the questions 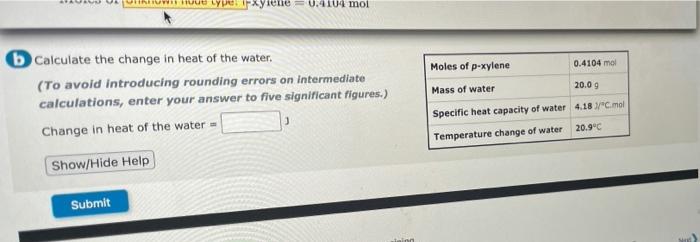
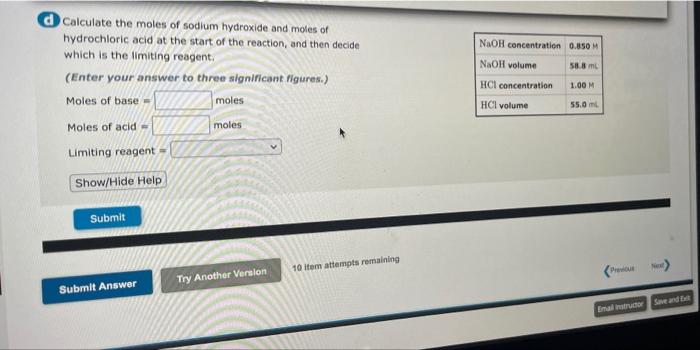
ULY pxyiene 0.410 mol Moles of p-xylene 0.4104 mol Mass of water 20.09 b Calculate the change in heat of the water. (To avoid introducing rounding errors on intermediate calculations, enter your answer to five significant figures.) Change in heat of the water - Show/Hide Help Specific heat capacity of water 4.18 "C.mol 20.9C Temperature change of water Submit Calculate the moles of sodium hydroxide and moles of hydrochloric acid at the start of the reaction, and then decide which is the limiting reagent. (Enter your answer to three significant ligures.) Moles of base- moles NaOH concentration 0.050 M NaOH volume 58.8 m HCl concentration 1.00 M HCl volume 55.0 ml Moles of acid moles Limiting reagent Show/Hide Help Submit 10 item attempts remaining Try Another Version Submit Answer Save and 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


