Answered step by step
Verified Expert Solution
Question
1 Approved Answer
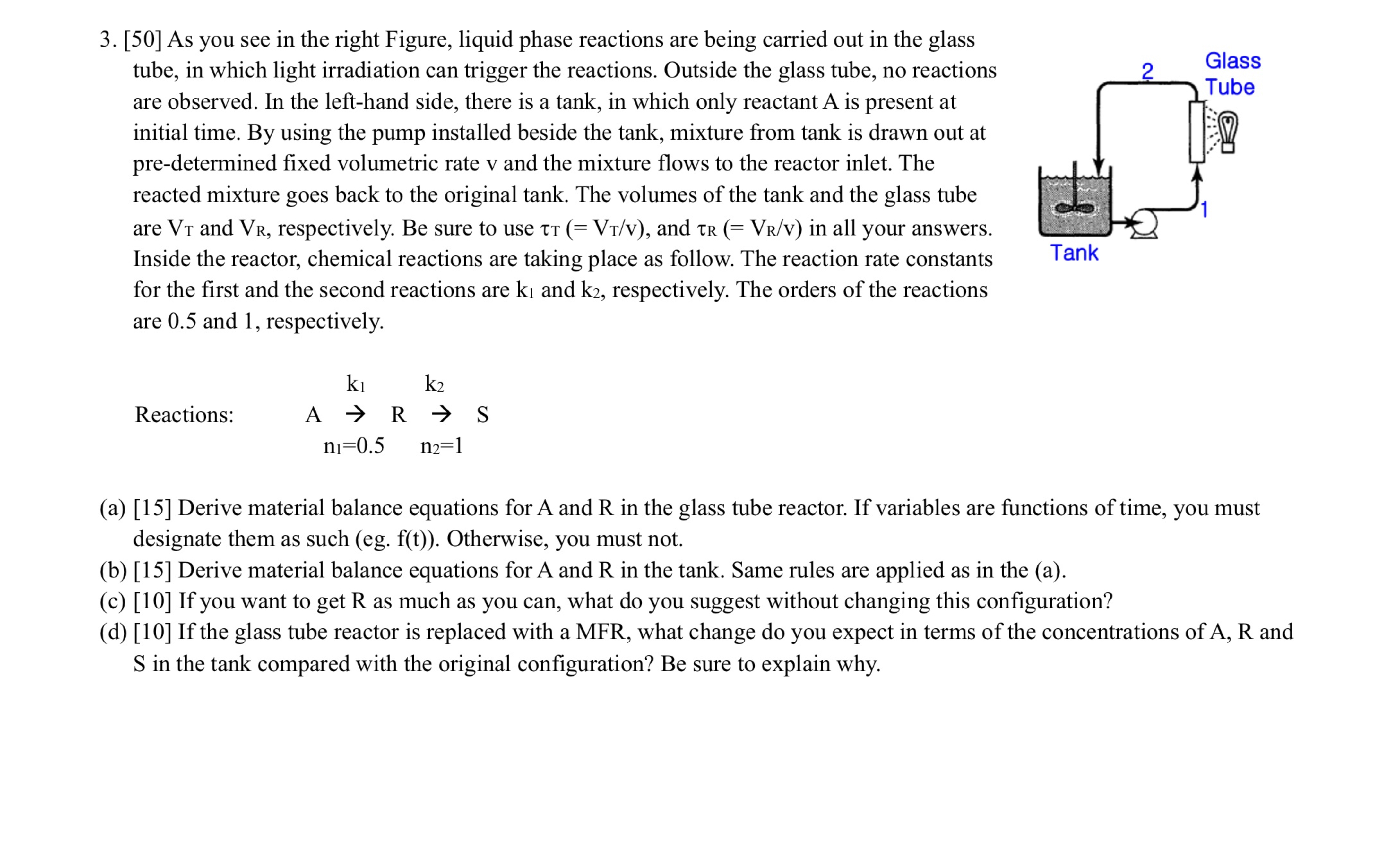
[ 5 0 ] As you see in the right Figure, liquid phase reactions are being carried out in the glass tube, in which light
As you see in the right Figure, liquid phase reactions are being carried out in the glass
tube, in which light irradiation can trigger the reactions. Outside the glass tube, no reactions
are observed. In the lefthand side, there is a tank, in which only reactant is present at
initial time. By using the pump installed beside the tank, mixture from tank is drawn out at
predetermined fixed volumetric rate and the mixture flows to the reactor inlet. The
reacted mixture goes back to the original tank. The volumes of the tank and the glass tube
are and respectively. Be sure to use and in all your answers.
Inside the reactor, chemical reactions are taking place as follow. The reaction rate constants
for the first and the second reactions are and respectively. The orders of the reactions
are and respectively.
Reactions:
a Derive material balance equations for A and in the glass tube reactor. If variables are functions of time, you must
designate them as such eg Otherwise, you must not.
b Derive material balance equations for A and in the tank. Same rules are applied as in the a
c If you want to get as much as you can, what do you suggest without changing this configuration?
d If the glass tube reactor is replaced with a MFR what change do you expect in terms of the concentrations of A R and
in the tank compared with the original configuration? Be sure to explain why.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


