Question
5. [20 Points] Consider the chemical reaction 3CO + 2.502 + 8 N XCO + YCO + ZO + 8N2 a. Determine the coefficients
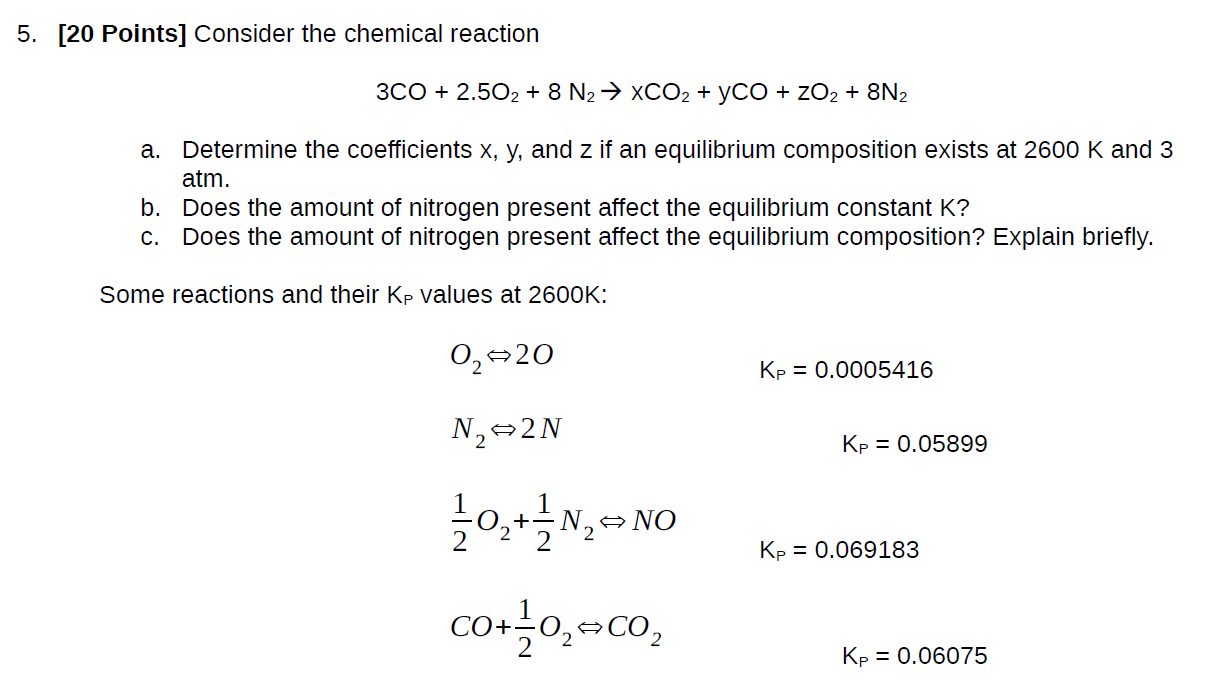
5. [20 Points] Consider the chemical reaction 3CO + 2.502 + 8 N XCO + YCO + ZO + 8N2 a. Determine the coefficients x, y, and z if an equilibrium composition exists at 2600 K and 3 atm. b. Does the amount of nitrogen present affect the equilibrium constant K? c. Does the amount of nitrogen present affect the equilibrium composition? Explain briefly. Some reactions and their Kp values at 2600K: 0 20 N 2N 21/0+1N NO NO CO+ 0 CO Kp = 0.0005416 Kp = 0.05899 Kp = 0.069183 Kp = 0.06075
Step by Step Solution
There are 3 Steps involved in it
Step: 1
a To determine the coefficients x y and z for the given chemical reaction at equilibrium we need to ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Organic Chemistry
Authors: Francis A. Carey
4th edition
0072905018, 978-0072905014
Students also viewed these Mechanical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



