Answered step by step
Verified Expert Solution
Question
1 Approved Answer
5 . 8 4 . Using the SRK equation of state ( or any other cubic equation of state ) to determine a specific volume
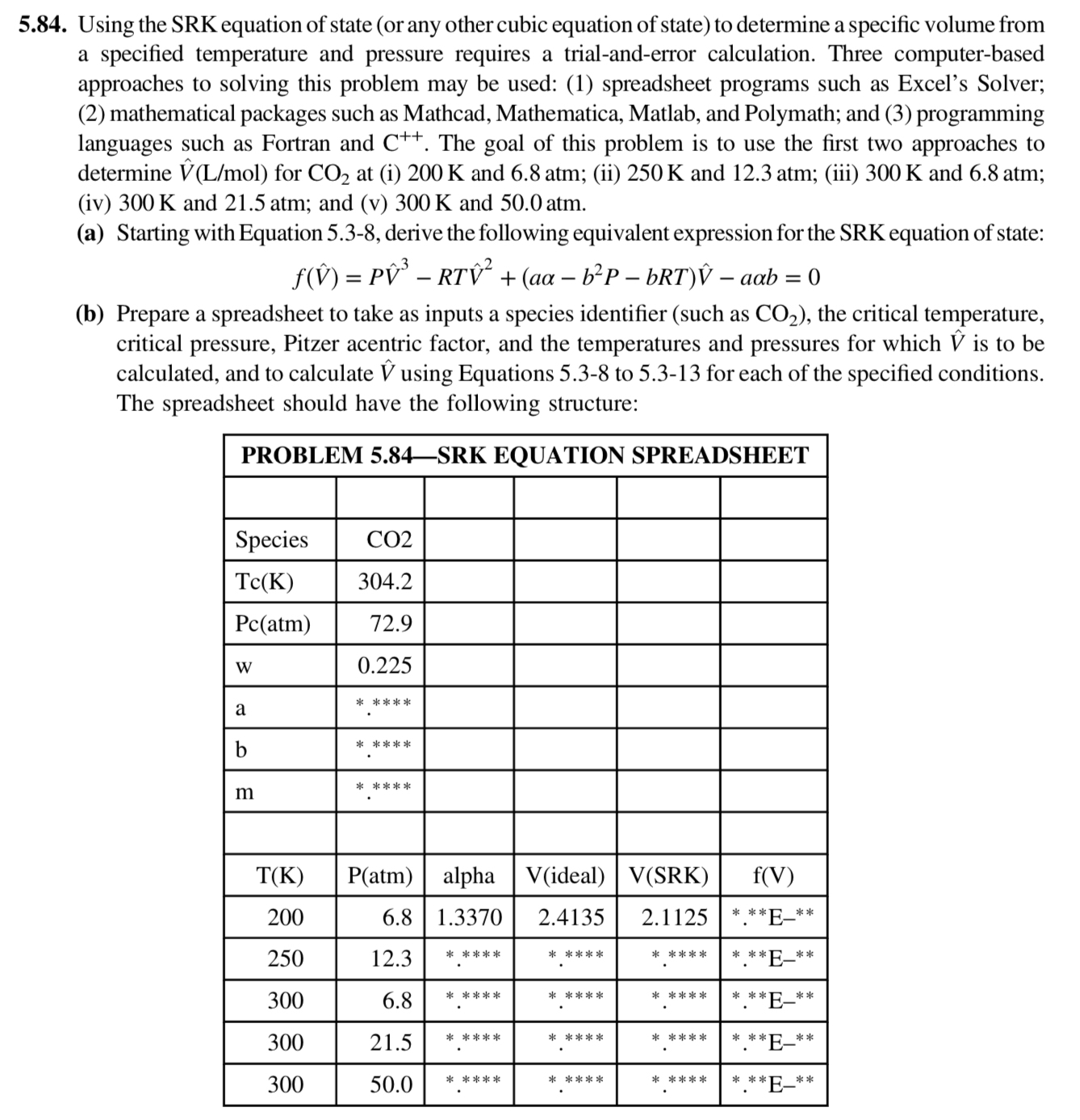
Using the SRK equation of state or any other cubic equation of state to determine a specific volume from a specified temperature and pressure requires a trialanderror calculation. Three computerbased approaches to solving this problem may be used: spreadsheet programs such as Excel's Solver; mathematical packages such as Mathcad, Mathematica, Matlab, and Polymath; and programming languages such as Fortran and The goal of this problem is to use the first two approaches to determine for at i and atm; ii and atm; iii and atm; iv and atm; and v and atm.
a Starting with Equation derive the following equivalent expression for the SRK equation of state:
PhatRThat
b Prepare a spreadsheet to take as inputs a species identifier such as the critical temperature, critical pressure, Pitzer acentric factor, and the temperatures and pressures for which hat is to be calculated, and to calculate hat using Equations to for each of the specified conditions. The spreadsheet should have the following structure:
tablePROBLEM SRK EQUATION SPREADSHEETSpecieswbalpha,Videal
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


