Answered step by step
Verified Expert Solution
Question
1 Approved Answer
5) Consider the following acid-base reactions in a and b: b) H :0: bl) (8 pts) label the acid, base, conjugate acid, and conjugate

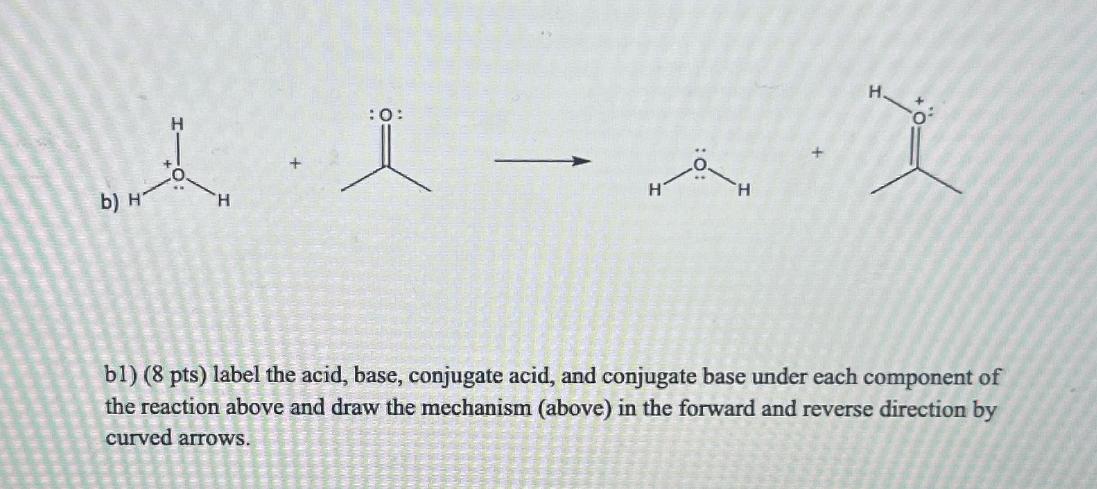

5) Consider the following acid-base reactions in a and b: b) H :0: bl) (8 pts) label the acid, base, conjugate acid, and conjugate base under each component of the reaction above and draw the mechanism (above) in the forward and reverse direction by curved arrows. B2) (8 pts) Predict the position of the equilibrium. In other words, is the forward direction favored or the reverse direction? Why? By what order of magnitude (how many times?). You must show your work below by consulting table 3.1
Step by Step Solution
There are 3 Steps involved in it
Step: 1
a CH3COO H2O CH3COOH OH b CH3COO HCl CH3COOH Cl For ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


