Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Entropy (ka/atom) 5. Consider the plot of the vibrational entropy Svib below for this material (remember 1043 K. What is the effect, if at
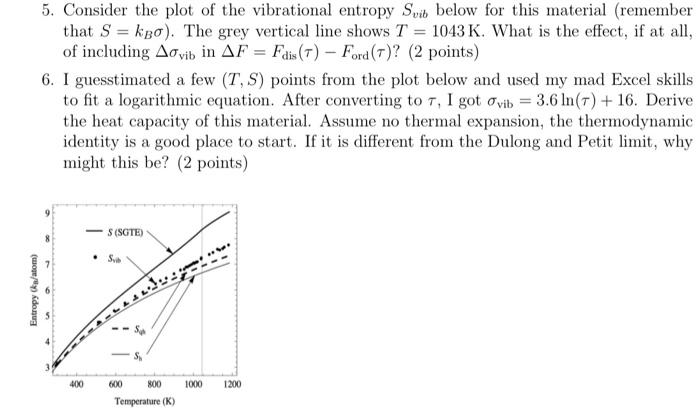
Entropy (ka/atom) 5. Consider the plot of the vibrational entropy Svib below for this material (remember 1043 K. What is the effect, if at all, = that Sko). The grey vertical line shows T of including Advib in AF = Fdis (7) Ford (7)? (2 points) 6. I guesstimated a few (T, S) points from the plot below and used my mad Excel skills to fit a logarithmic equation. After converting to 7, I got ovib = 3.6 ln(7) + 16. Derive the heat capacity of this material. Assume no thermal expansion, the thermodynamic identity is a good place to start. If it is different from the Dulong and Petit limit, why might this be? (2 points) 400 S (SGTE) Syb 600 Sp 800 Temperature (K) 1000 1200
Step by Step Solution
★★★★★
3.37 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1
5 The inclusion of Aovib in AF Fdis 7 Ford 7 would increase the vibrational entropy of the material at any given temperature This is because the Aovib ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


