Question
5. Determine the limiting reagent, and calculate theoretical yield and percentage (%) yield for tert-butyl chloride from the following data. Chemical Tert-butanol Tert-butyl chloride
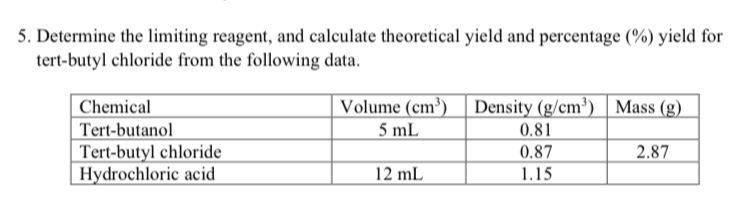
5. Determine the limiting reagent, and calculate theoretical yield and percentage (%) yield for tert-butyl chloride from the following data. Chemical Tert-butanol Tert-butyl chloride Hydrochloric acid Volume (cm) 5 mL 12 mL Density (g/cm) 0.81 0.87 1.15 Mass (g) 2.87
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Determining the Limiting Reagent To determine the limiting reagent in a chemical reaction we need to ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemistry Principles And Practice
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
3rd Edition
0534420125, 9780534420123
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



