When methyl bromide reacts with hydroxide ion in solution, methyl alcohol and bromide ion form. Determine the
Question:
When methyl bromide reacts with hydroxide ion in solution, methyl alcohol and bromide ion form.![]()
Determine the rate law and evaluate the rate constant from the experimental data.![Experiment 1 2 3 Initial Concentration (M) [CH3Br] [OH-] 0.050 0.010 0.080 0.020 0.080 0.010 Initial Rate](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1704/3/4/3/219659636b3ea00e1704343219529.jpg)
Strategy
An information flow diagram helps to illustrate the problem-solving strategy. First, calculate relative concentrations and rates. Next, find experiments in which only one concentration varies, and compare the changes in concentrations and changes in rates to deduce the order of that reactant. Finally, use the rate law and the experimental data to calculate the rate constant.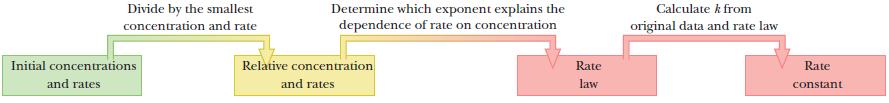
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:





