When methyl bromide reacts with hydroxide ion, methyl alcohol and bromide ion form. CH3Br + OH CH3OH
Question:
When methyl bromide reacts with hydroxide ion, methyl alcohol and bromide ion form.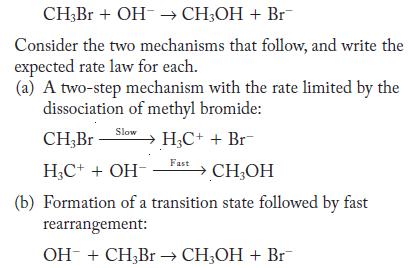
Transcribed Image Text:
CH3Br + OH CH3OH + Br Consider the two mechanisms that follow, and write the expected rate law for each. (a) A two-step mechanism with the rate limited by the dissociation of methyl bromide: Slow CH,Br HC++ Br HC+ + OH- (b) Formation of a transition state followed by fast rearrangement: OH + CH3Br CH3OH + Br Fast CHOH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
You are correctThe reaction between methyl bromide CHBr and hydroxide ion OH is a classic example of a nucleophilic substitution reactionwhere the hyd...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
When methyl bromide reacts with hydroxide ion in solution, methyl alcohol and bromide ion form. Determine the rate law and evaluate the rate constant from the experimental data. Strategy An...
-
When magnesium metal is burned in air (Figure 3.6), two products are produced. One is magnesium oxide, MgO. The other is the product of the reaction of Mg with molecular nitrogen, magnesium nitride....
-
The reaction between ethyl bromide (C2H5Br) and hydroxide ion in ethyl alcohol at 330 K, C2H5Br(alc) + OH (alc) -- C2H5OH(I) + Br(aIc), is first order each in ethyl bromide and hydroxide ion. When...
-
Explain why entrepreneurial firms are often in a strong position to use combination strategies.
-
Find V1 in the network infigure. Vx + ww 10 kn 5 kn V1 +)25 V +1
-
What is the financial managers objective in evaluating pro forma statements?
-
Will I be able to refrain from making negative statements about people I worked with in the past? LO.1
-
Stanley Furniture Company is a Virginia-based furniture manufacturer. For each of the following firstyear transactions, indicate whether net cash inflows (outflows) from operating activities (NCFO),...
-
Save Antwer Question 1 2 points Kent university currently has manual student information system. The narrative of this system is presented as follow: Narrative of the manual SIS: The students...
-
Ethyl chloride decomposes to form ethylene and hydrogen chloride at 437 K. The reaction takes place in a 4.0-L container and is monitored by measuring the time needed for the hydrogen chloride to...
-
Two reactions have activation energies of 45 and 40 kJ/mol, respectively. Which reaction shows the greater increase in rate with an increase in temperature?
-
Vale has been in business for some years. The following balances were brought forward in his books of account as at 1 January 2020: During the year to 31 December 2020 the following transactions took...
-
Fineas Co. use the Job Order Costing system to determine product costs. Before entering 2020, the company has created a production budget, with an estimated total manufacturing overhead of $...
-
Define what a market value is? What are three major principles of investing funds? How does the federal government control the money supply? An investor purchases a 10-year U.S. Treasury note and...
-
1. Suppose we have two alternative designs, each of which yields a different present value of the total lifetime cost: the first is $1604 and the second is $1595. Verify that the present value of the...
-
Sometimes when we are asked for a linear model, the information that we are given is data about a scenario. In these cases we have to use Excel to generate a trendline. There is a video in this...
-
1. Purpose Explain 3 points from the Introduction section as to why this study is important. How did this study build on the existing literature in this area? 2. Participants Outline at least 2...
-
Although Hückel's rule (Section 14.7) strictly applies only to monocyclic compounds, it does appear to have application to certain bicyclic compounds, if one assumes use of resonance...
-
Hardin Services Co. experienced the following events in 2016: 1. Provided services on account. 2. Collected cash for accounts receivable. 3. Attempted to collect an account and, when unsuccessful,...
-
The L-shaped frame is made from two segments, each of length Land flexural stiffness EI. If it is subjected to the uniform distributed load, determine the vertical displacement of point B. Using...
-
The L-shaped frame is made from two segments, each of length Land flexural stiffness EI. If it is subjected to the uniform distributed load, determine the horizontal displacement of the end C. Use...
-
Determine the vertical deflection at C. The cross-sectional area and moment of inertia of each segment is shown in the figure. Take E = 200 GPa. Assume A is a fixed support. Including the effect of...
-
What general conclusions can you draw about your companys liquidity, solvency and productivity based on your ratio calculations. Working Capital 2017 = $9,994 M 2016 = $10,673 M Current Ratio 2017 =...
-
Tami Tyler opened Tami's Creations, Incorporated, a small manufacturing company, at the beginning of the year. Getting the company through its first quarter of operations placed a considerable strain...
-
5. The current spot exchange rate is 0.95/$ and the three-month forward rate is 0.91/$. Based on your analysis of the exchange rate, you are pretty confident that the spot exchange rate will be...

Study smarter with the SolutionInn App


