Ethyl chloride decomposes to form ethylene and hydrogen chloride at 437 K. The reaction takes place in
Question:
Ethyl chloride decomposes to form ethylene and hydrogen chloride at 437 K.![]()
The reaction takes place in a 4.0-L container and is monitored by measuring the time needed for the hydrogen chloride to react with a known amount of base.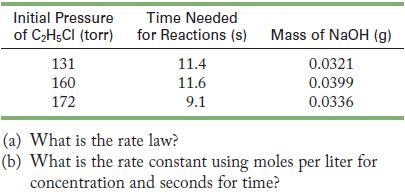
Transcribed Image Text:
CHCl(g) CH4(g) + HCl(g) -
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
a first ...View the full answer

Answered By

Simon kingori
I am a tier-one market researcher and content developer who has been in this field for the last six years. I’ve run the freelancing gamut; from market research, data mining and SEO/SMM to copywriting, Content Development, you name it, I’ve done it. I’m extremely motivated, organized and disciplined – you have to be to work from home. My experience in Freelancing is invaluable- but what makes me a cut above the rest is my passion to deliver quality results to all my clients- it’s important to note, I've never had a dissatisfied client. Backed by a Masters degree in Computer Science from MOI university, I have the required skill set and burning passion and desire to deliver the best results for my clients. This is the reason why I am a cut above the rest. Having taken a Bsc. in computer science and statistics, I deal with all round fields in the IT category. It is a field i enjoy working in as it is dynamic and new things present themselves every day for research and exploration.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
A chemical reaction takes place in a container of cross-sectional area 50.0 cm/. As a result of the reaction, a piston is pushed out through 15 cm against an external pressure of 121 kPa. Calculate...
-
Explain the role of crocodiles in a malaria region, include all components of the food chain involved and provide suitable examples.
-
The following are two independent situations. (Credit account titles are automatically indented when amount is entered. Do not indent manually. Record journal entries in the order prented in the...
-
In the context of online marketing communications, briefly explain what viral marketing is. Is it a worth-while pursuit for marketing organizations?
-
Calculate the power absorbed by the dependent source in the circuit infigure. 4 K 2 kn +- 12 V 1.5 Vx + 6 3 kn 3
-
What are the two basic weaknesses of the simplified approaches to preparing pro forma statements?
-
Will I be able to give examples of how I have been a team player? LO.1
-
A laser-materials-processing apparatus encloses a sample in the form of a disk of diameter D = 25 mm and thickness w = 1 mm. The sample has a diffuse surface for which the spectral distribution of...
-
QUESTION 1 The percentage of demand that can be satisfied is Type I service level Type Il service level Type Ill service level None of the above
-
The following experimental data were obtained in a study of the kinetics of the gas-phase formation of nitrosyl bromide at 791 K. Determine the rate law and the rate constant from the data.
-
When methyl bromide reacts with hydroxide ion, methyl alcohol and bromide ion form. CH3Br + OH CH3OH + Br Consider the two mechanisms that follow, and write the expected rate law for each. (a) A...
-
The magnitude of the electrostatic force between two identical ions that are separated by u distance of 5.0 x 1010 m is 3.7 x 109 N. (a) What is the charge of each ion? (b) How many electrons are...
-
Each of the accompanying graphs shows a do plot of data from three separate random samples for each of the four graphs, indicate whether you think that the basic assumptions for single-factor ANOVA...
-
Program Milestones Milestone #1 - Selection GUI - Create Account/Login/Cancel Obtain a copy of Eclipse and complete "Getting Started in Eclipse". Create your project in Eclipse and the package and...
-
= The momentum transfer is q = ph - Ph, where p is the hadron momentum after the collision. This relationship holds for the time as well as the space components, i.e., for the 4-vectors. Thus, we...
-
When should HR be the interviewer, and when should a hiring manager or co-workers be involved? Should reference checking be done before or after the interview? Would you ask during the interview any...
-
1. Monoclean Company manufactures a single product, Glamour. The standard cost specification sheet shows the following standards for one unit of Glamour: 8 kg of material M @ $6.5 per kg $52 4 hours...
-
(a) In 1960 T. Katz (Columbia University) showed that cyclooctatetraene adds two electrons when treated with potassium metal and forms a stable, planar dianion, C8H82- (as the dipotassium salt): Use...
-
Q1) What is the a3 Value Q2) What is the a7 Value Q3) What is the a4 Value Q4) What is the b3 Value Q5) What is the b2 Value Q6) What is the sign of 2nd constraint? A pastry chef at a bakery wants to...
-
Determine the vertical deflection at C. The cross-sectional area and moment of inertia of each segment is shown in the figure. Take E = 200 GPa. Assume A is a fixed support. Use the method of virtual...
-
Determine the horizontal displacement of point C. EI is constant. Using Castiglianos theorem.
-
Determine the horizontal displacement of point C. EI is constant. Use the method of virtual work.
-
business law A partner may actively compete with the partnership True False
-
A company provided the following data: Selling price per unit $80 Variable cost per unit $45 Total fixed costs $490,000 How many units must be sold to earn a profit of $122,500?
-
Suppose a 10-year, 10%, semiannual coupon bond with a par value of $1,000 is currently selling for $1,365.20, producing a nominal yield to maturity of 7.5%. However, it can be called after 4 years...

Study smarter with the SolutionInn App


