Answered step by step
Verified Expert Solution
Question
1 Approved Answer
5. Determine the standard electrode potential of this half-cell reaction: Hg=Hg2++2e ...where Hg is still metallic/elemental mercury (Hg (liquid)). Isotope Information (for Geochron and Stable

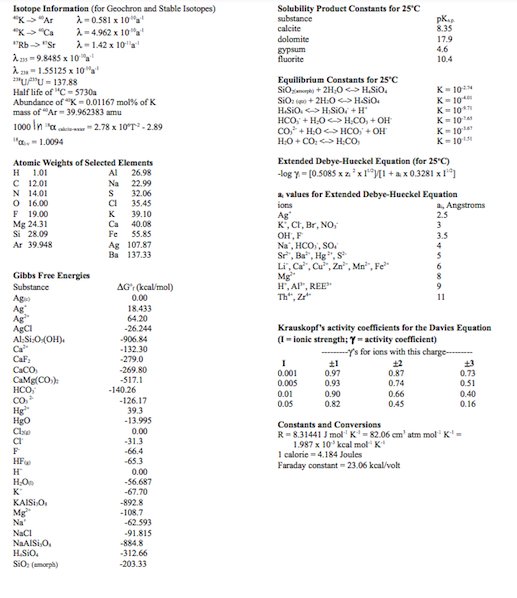 5. Determine the standard electrode potential of this half-cell reaction: Hg=Hg2++2e ...where Hg is still metallic/elemental mercury (Hg (liquid)). Isotope Information (for Geochron and Stable Isotopes) 23=9.84851030a123=1.551251014a1231U+15U=137.88Halflifeof11C=5730aAbundanceofK=0.01167mol%ofKmassof20Ar=39.962383amu1000ln1"abewa=2.7810T22.8910x=1.0094 Extended Debye-Hucekel Equation (for 25C ) log=[0.5085z2119][1+a0.3281I16] Krauskopf's aetivity coefficients for the Davies Equation ( I = ionie strength; = activity coefficient) Cosstants and Cenversions R=8.31441Jmol1K1=82.06cm3atmmol1K1=1.987101kcalmol4K1Icaloric=4.184JoulesFaradayconstant=23.06kcal/volt 5. Determine the standard electrode potential of this half-cell reaction: Hg=Hg2++2e ...where Hg is still metallic/elemental mercury (Hg (liquid)). Isotope Information (for Geochron and Stable Isotopes) 23=9.84851030a123=1.551251014a1231U+15U=137.88Halflifeof11C=5730aAbundanceofK=0.01167mol%ofKmassof20Ar=39.962383amu1000ln1"abewa=2.7810T22.8910x=1.0094 Extended Debye-Hucekel Equation (for 25C ) log=[0.5085z2119][1+a0.3281I16] Krauskopf's aetivity coefficients for the Davies Equation ( I = ionie strength; = activity coefficient) Cosstants and Cenversions R=8.31441Jmol1K1=82.06cm3atmmol1K1=1.987101kcalmol4K1Icaloric=4.184JoulesFaradayconstant=23.06kcal/volt
5. Determine the standard electrode potential of this half-cell reaction: Hg=Hg2++2e ...where Hg is still metallic/elemental mercury (Hg (liquid)). Isotope Information (for Geochron and Stable Isotopes) 23=9.84851030a123=1.551251014a1231U+15U=137.88Halflifeof11C=5730aAbundanceofK=0.01167mol%ofKmassof20Ar=39.962383amu1000ln1"abewa=2.7810T22.8910x=1.0094 Extended Debye-Hucekel Equation (for 25C ) log=[0.5085z2119][1+a0.3281I16] Krauskopf's aetivity coefficients for the Davies Equation ( I = ionie strength; = activity coefficient) Cosstants and Cenversions R=8.31441Jmol1K1=82.06cm3atmmol1K1=1.987101kcalmol4K1Icaloric=4.184JoulesFaradayconstant=23.06kcal/volt 5. Determine the standard electrode potential of this half-cell reaction: Hg=Hg2++2e ...where Hg is still metallic/elemental mercury (Hg (liquid)). Isotope Information (for Geochron and Stable Isotopes) 23=9.84851030a123=1.551251014a1231U+15U=137.88Halflifeof11C=5730aAbundanceofK=0.01167mol%ofKmassof20Ar=39.962383amu1000ln1"abewa=2.7810T22.8910x=1.0094 Extended Debye-Hucekel Equation (for 25C ) log=[0.5085z2119][1+a0.3281I16] Krauskopf's aetivity coefficients for the Davies Equation ( I = ionie strength; = activity coefficient) Cosstants and Cenversions R=8.31441Jmol1K1=82.06cm3atmmol1K1=1.987101kcalmol4K1Icaloric=4.184JoulesFaradayconstant=23.06kcal/volt Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


