Question
5. How long did it take for the reaction to go to completion? If the reaction did not go to completion, write that it did
5. How long did it take for the reaction to go to completion? If the reaction did not go to completion, write that it did not go to completion.
How do I interpret the 1H NMR spectra? Look carefully at the spectra of the starting material and the product; in a mixture of these two things, peaks from both will be present in the 1H NMR spectrum.
Most of the 1H NMR spectra contain a mixture of both the starting material and the product. In these spectra, two peaks are integrated, one from the starting material and one from the product
. In a mixture of two things in 1H NMR, you need to calculate a ratio of the two things in solution. Based on the spectra of the starting material and product, determine what the integration of the two peaks should be for a pure compound.
Divide the integration of the mixture by what the integration should be. This will give you a ratio of the two compounds. For example, if the integration should be 3 but in the mixture it is 1, then take 1/3 to get 0.333 for that one peak.
Convert the ratio to a decimal. For example, if you have a 1:1 mixture of compounds, then that equates to 0.50 of product and 0.50 of starting material (a 50:50 mixture).
You need to do this for every spectrum that has both starting material and product in it. Note: If the reaction goes to completion (only product and no starting material), then you have 100% product; all of the starting material converted to product.
Please help do this i am confused on what i am supposed to do to tell if the spectra is complete or not. could you please write out your work for each spectra and show what time it was completion. Thank you
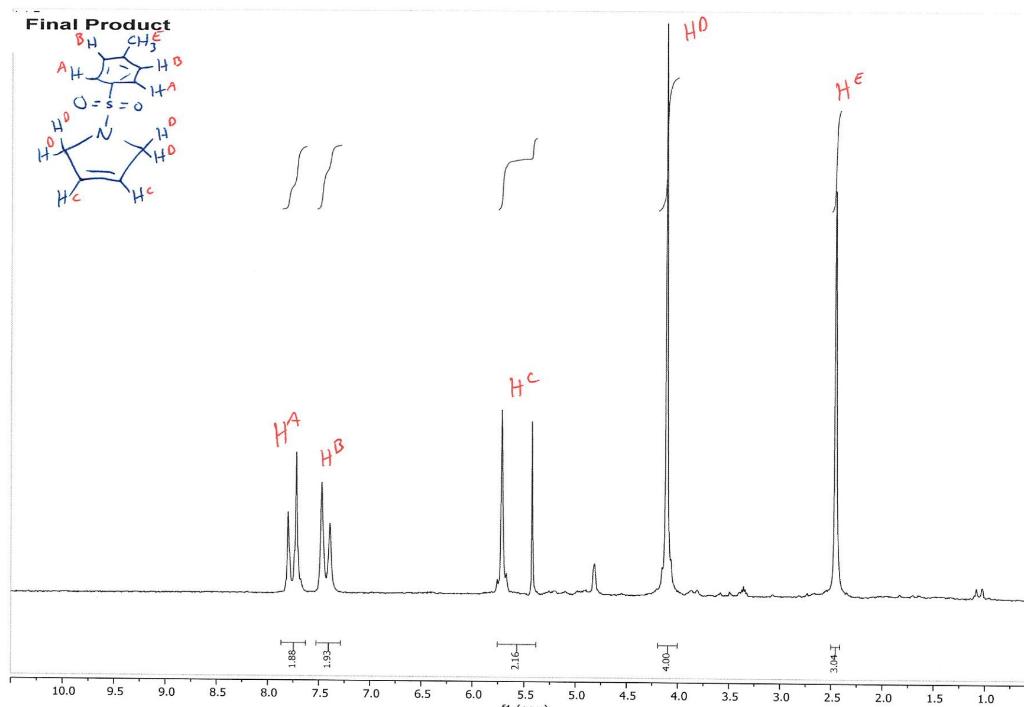
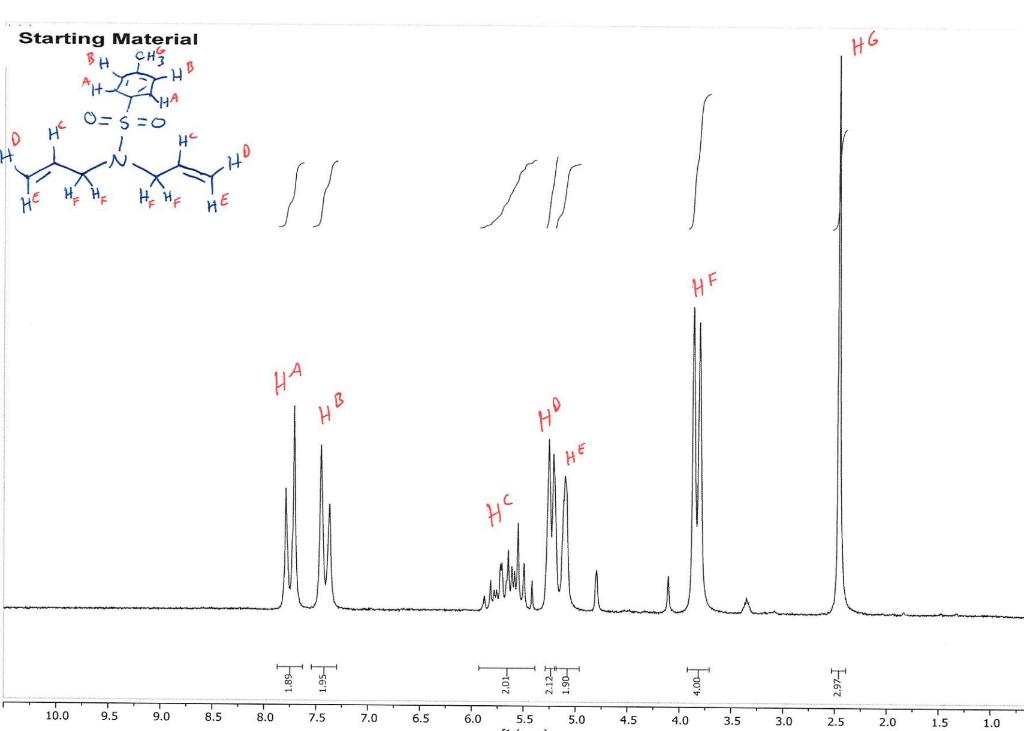
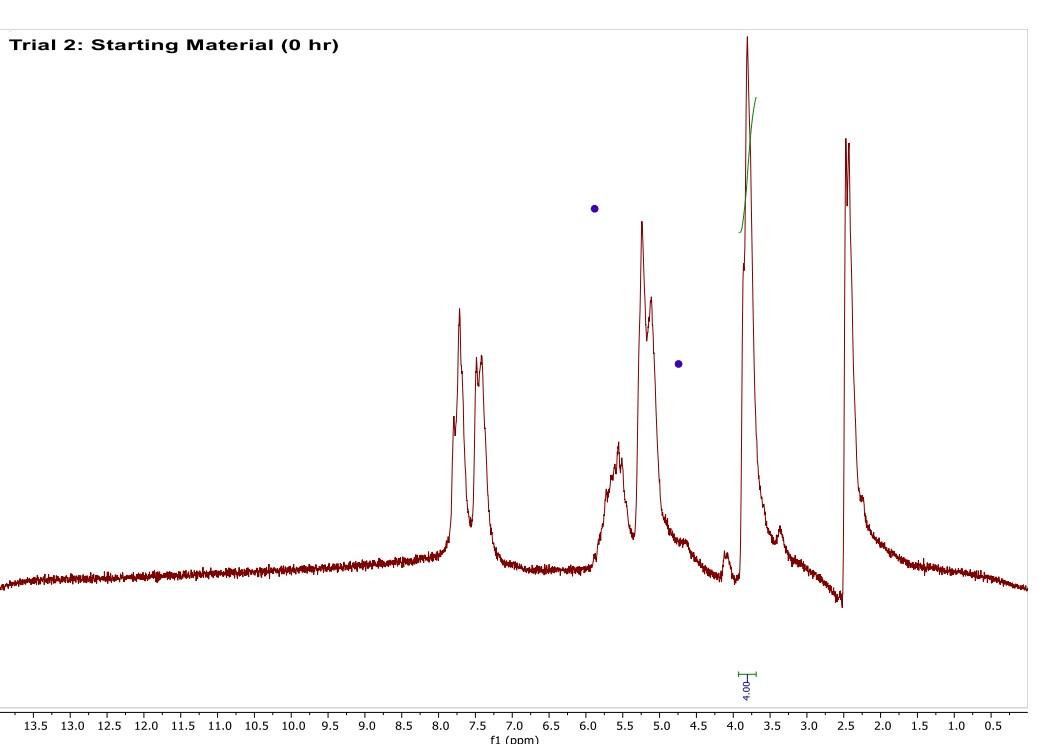
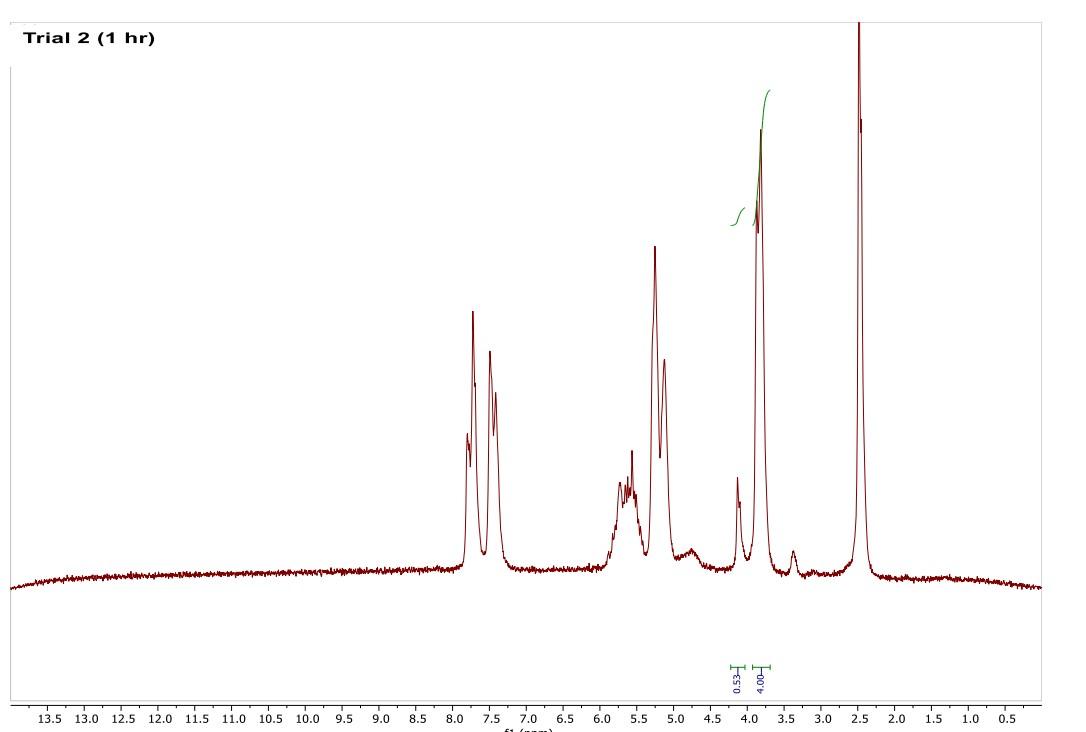
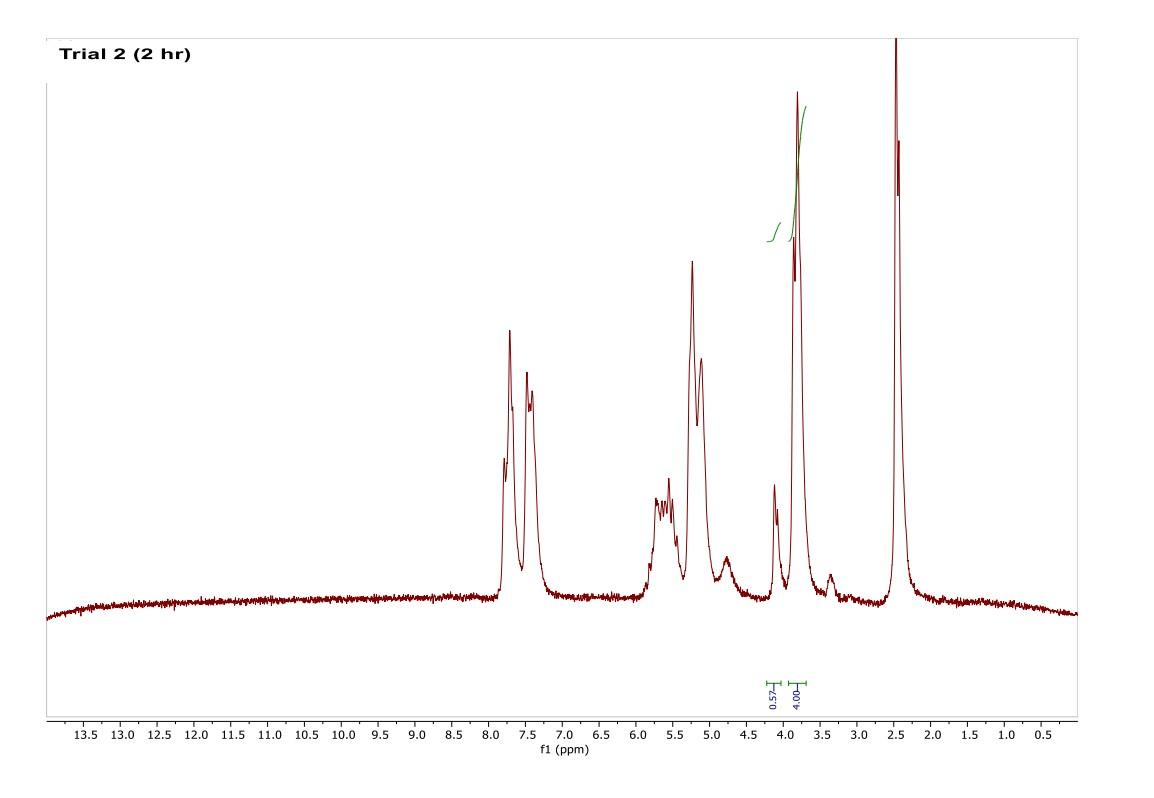
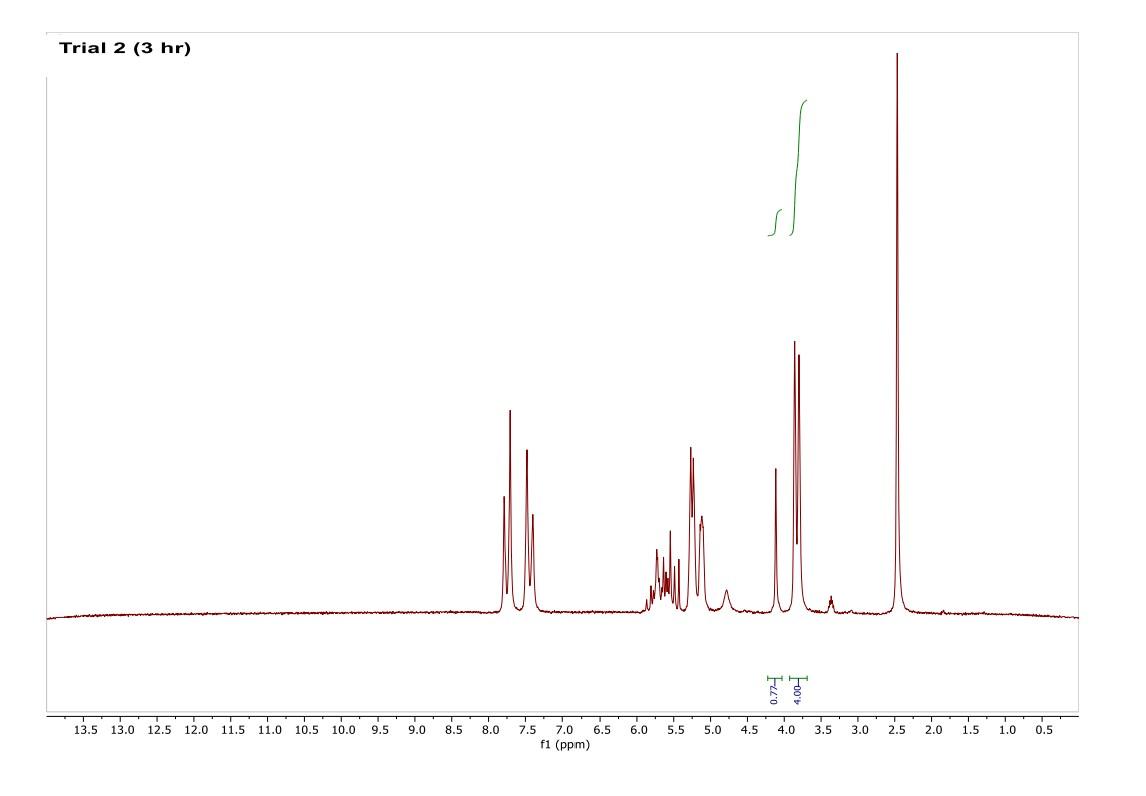
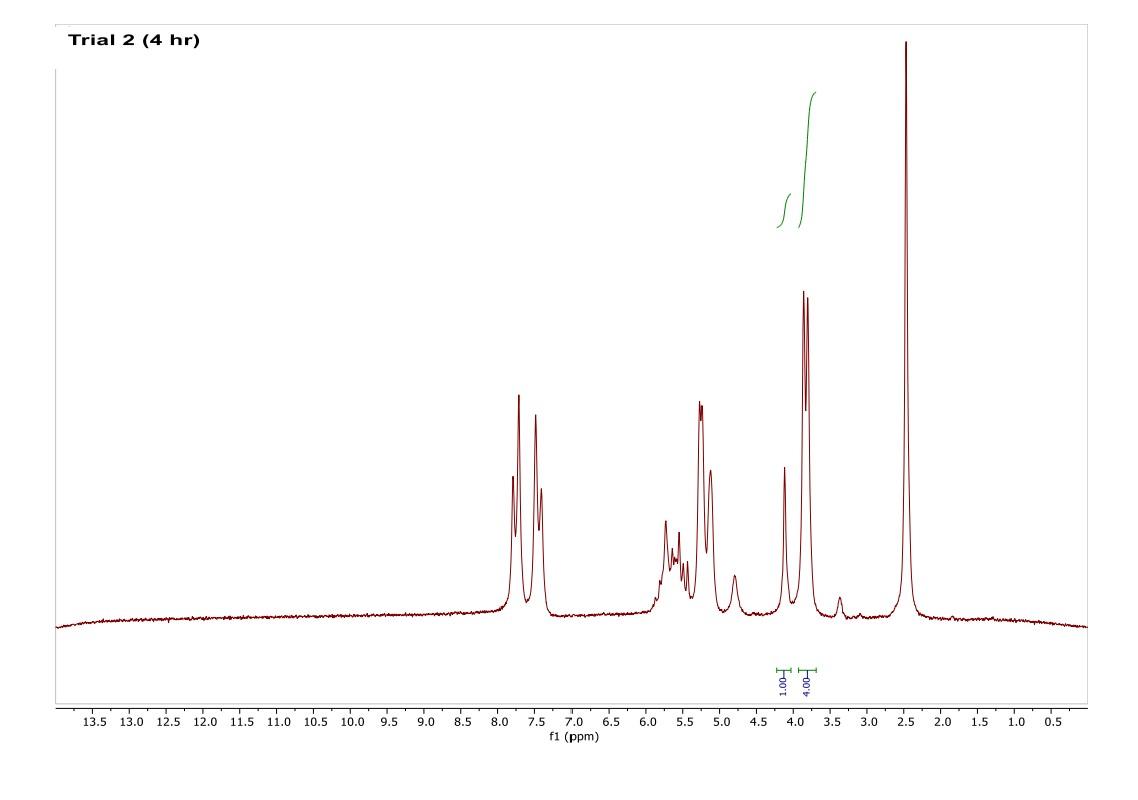
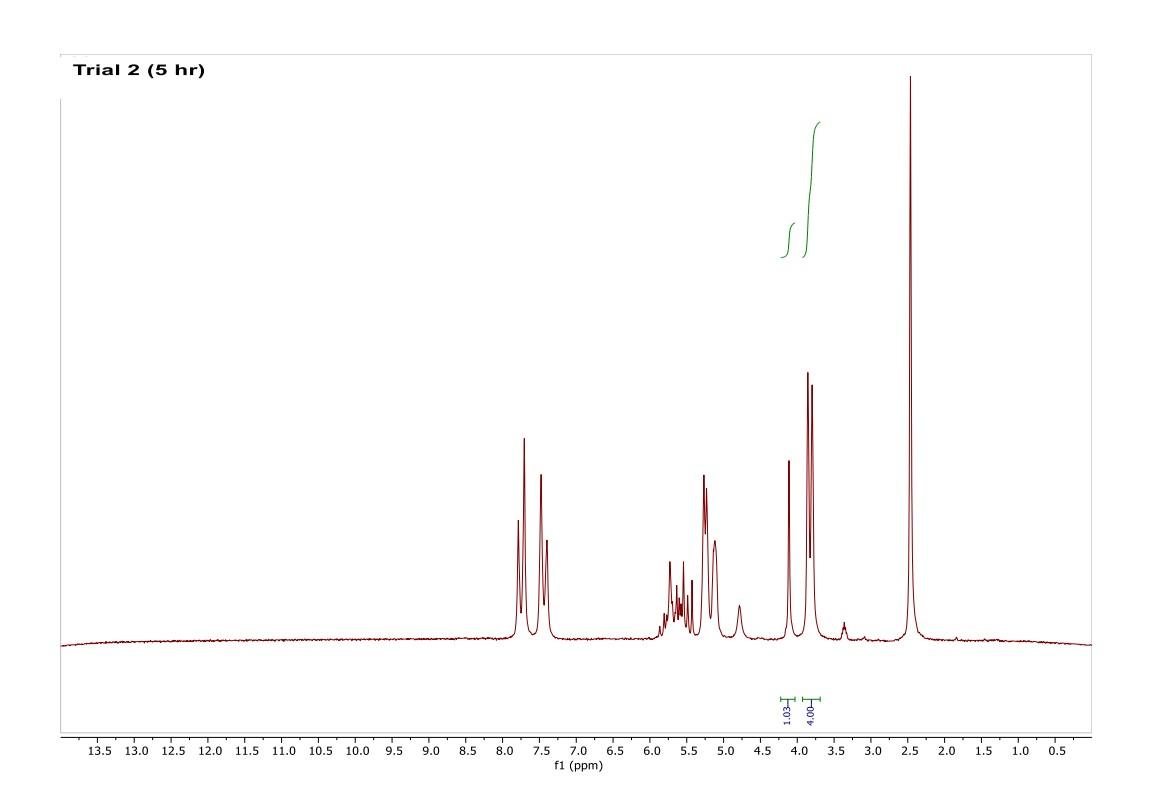
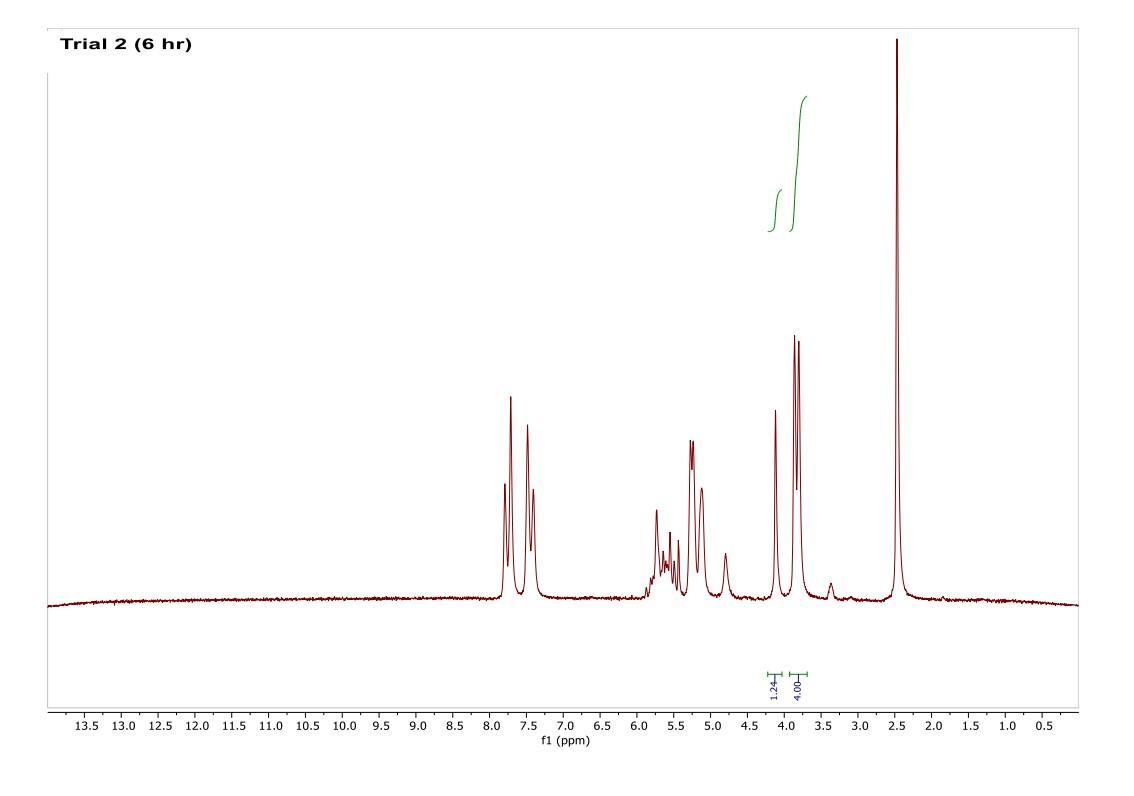
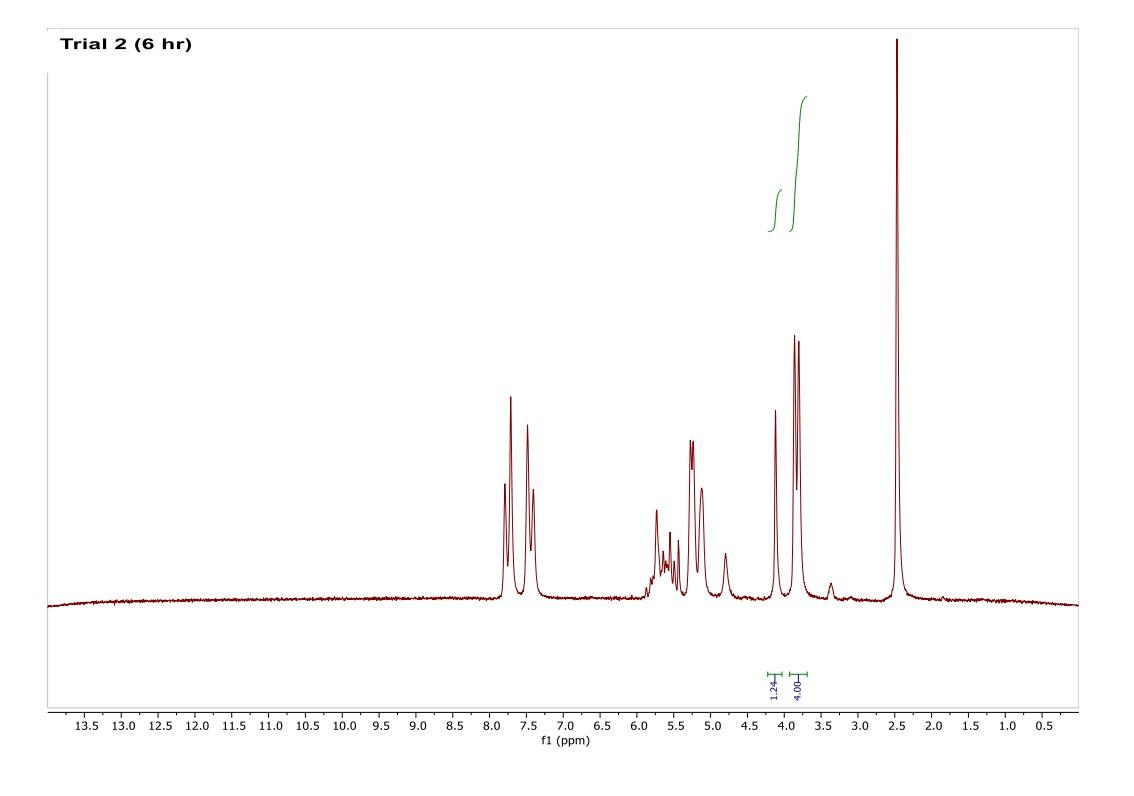
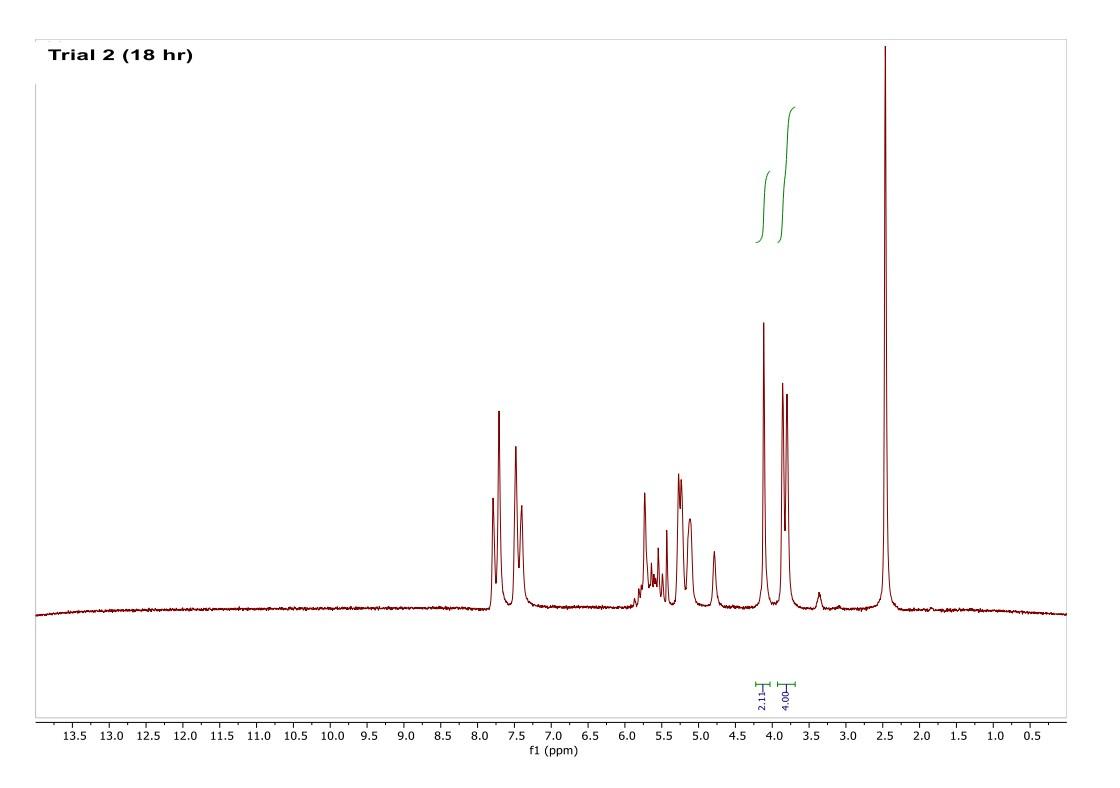 You need to do this for every spectrum that has both starting material and product in it. Note: If the reaction goes to completion (only product and no starting material), then you have 100% product; all of the starting material converted to product. Please help do this i am confused on what i am supposed to do to tell if the spectra is complete or not. could you please write out your work for each spectra and show what time it was completion. Thank you
You need to do this for every spectrum that has both starting material and product in it. Note: If the reaction goes to completion (only product and no starting material), then you have 100% product; all of the starting material converted to product. Please help do this i am confused on what i am supposed to do to tell if the spectra is complete or not. could you please write out your work for each spectra and show what time it was completion. Thank you
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


