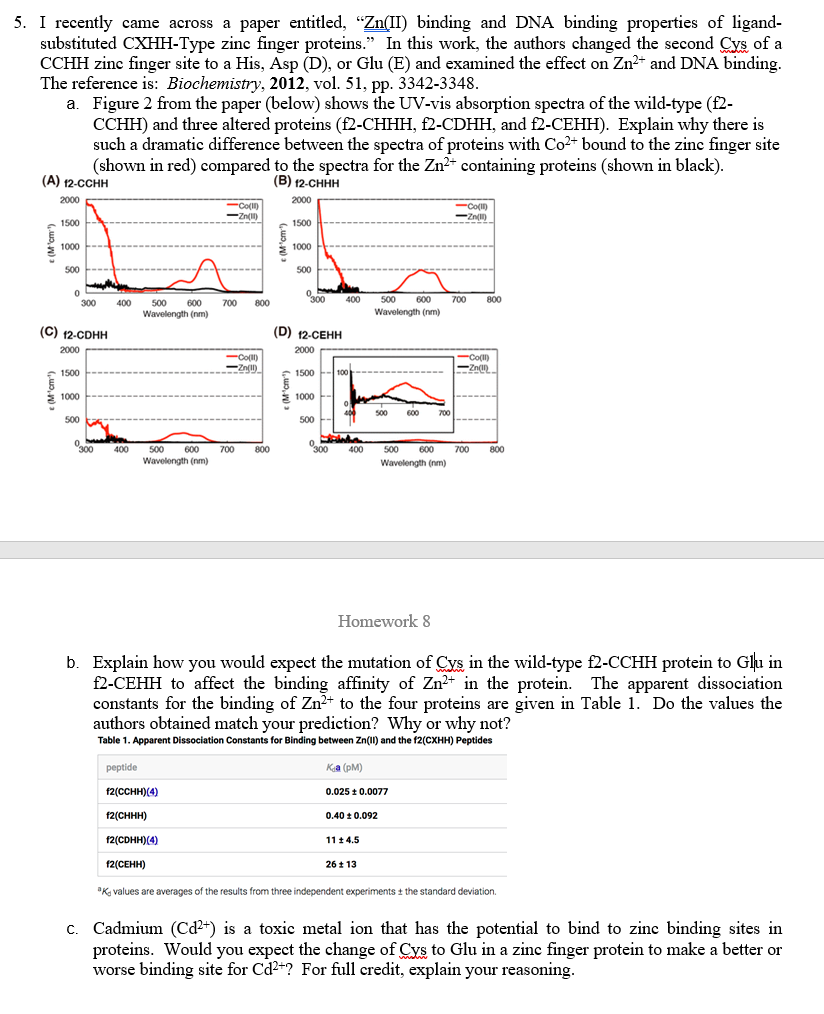
5. I recently came across a paper entitled, Zn(II) binding and DNA binding properties of ligand- substituted CXHH-Type zinc finger proteins. In this work, the authors changed the second Cys of a CCHH zinc finger site to a His, Asp (D), or Glu (E) and examined the effect on Zn+ and DNA binding. The reference is: Biochemistry, 2012, vol. 51, pp. 3342-3348. a. Figure 2 from the paper (below) shows the UV-vis absorption spectra of the wild-type (f2- CCHH) and three altered proteins (f2-CHHH, f2-CDHH, and f2-CEHH). Explain why there is such a dramatic difference between the spectra of proteins with Co2+ bound to the zinc finger site (shown in red) compared to the spectra for the Zn2+ containing proteins (shown in black). (A) 12-CCHH (B) 12-CHHH 2000 2000 Co(II) -Zn(1) -Co(IT) Zn(1) 1500 1500 (Mom") 1000 1000 500 500 0 0 0 300 400 400 700 300 800 500 600 Wavelength (nm) 700 800 500 600 Wavelength (nm) (C) 2-CDHH (D) 12-CEHH ) 2000 2000 -Coll) ( - Zn(1) -Co(II) -Zn() 1500 (Mom") 1000 1500-100 $ 6 - 1000 400 500 500 600 700 500 000 300 0 Mehr 300 400 400 700 800 700 800 500 600 Wavelength (nm) 500 600 Wavelength (nm) Homework 8 b. Explain how you would expect the mutation of Cys in the wild-type f2-CCHH protein to Glu in f2-CEHH to affect the binding affinity of Zn2+ in the protein. The apparent dissociation constants for the binding of Zn+ to the four proteins are given in Table 1. Do the values the authors obtained match your prediction? Why or why not? Table 1. Apparent Dissociation Constants for Binding between Zn(II) and the f2(CXHH) Peptides peptide Kia (PM) ) f2(CCHH)(4) 0.025 0.0077 f2(CHHH) 0.40 0.092 f2(CDHH)(4) 11 4.5 () 12() 26 1 13 Ky values are averages of the results from three independent experiments the standard deviation - . C. Cadmium (Cd2+) is a toxic metal ion that has the potential to bind to zinc binding sites in proteins. Would you expect the change of Cys to Glu in a zinc finger protein to make a better or worse binding site for Cd2+? For full credit, explain your reasoning. 5. I recently came across a paper entitled, Zn(II) binding and DNA binding properties of ligand- substituted CXHH-Type zinc finger proteins. In this work, the authors changed the second Cys of a CCHH zinc finger site to a His, Asp (D), or Glu (E) and examined the effect on Zn+ and DNA binding. The reference is: Biochemistry, 2012, vol. 51, pp. 3342-3348. a. Figure 2 from the paper (below) shows the UV-vis absorption spectra of the wild-type (f2- CCHH) and three altered proteins (f2-CHHH, f2-CDHH, and f2-CEHH). Explain why there is such a dramatic difference between the spectra of proteins with Co2+ bound to the zinc finger site (shown in red) compared to the spectra for the Zn2+ containing proteins (shown in black). (A) 12-CCHH (B) 12-CHHH 2000 2000 Co(II) -Zn(1) -Co(IT) Zn(1) 1500 1500 (Mom") 1000 1000 500 500 0 0 0 300 400 400 700 300 800 500 600 Wavelength (nm) 700 800 500 600 Wavelength (nm) (C) 2-CDHH (D) 12-CEHH ) 2000 2000 -Coll) ( - Zn(1) -Co(II) -Zn() 1500 (Mom") 1000 1500-100 $ 6 - 1000 400 500 500 600 700 500 000 300 0 Mehr 300 400 400 700 800 700 800 500 600 Wavelength (nm) 500 600 Wavelength (nm) Homework 8 b. Explain how you would expect the mutation of Cys in the wild-type f2-CCHH protein to Glu in f2-CEHH to affect the binding affinity of Zn2+ in the protein. The apparent dissociation constants for the binding of Zn+ to the four proteins are given in Table 1. Do the values the authors obtained match your prediction? Why or why not? Table 1. Apparent Dissociation Constants for Binding between Zn(II) and the f2(CXHH) Peptides peptide Kia (PM) ) f2(CCHH)(4) 0.025 0.0077 f2(CHHH) 0.40 0.092 f2(CDHH)(4) 11 4.5 () 12() 26 1 13 Ky values are averages of the results from three independent experiments the standard deviation - . C. Cadmium (Cd2+) is a toxic metal ion that has the potential to bind to zinc binding sites in proteins. Would you expect the change of Cys to Glu in a zinc finger protein to make a better or worse binding site for Cd2+? For full credit, explain your reasoning







