Answered step by step
Verified Expert Solution
Question
1 Approved Answer
5. In one experiment a student wanted to determine the amount of F- in a mouth wash sample from the shop. The students first
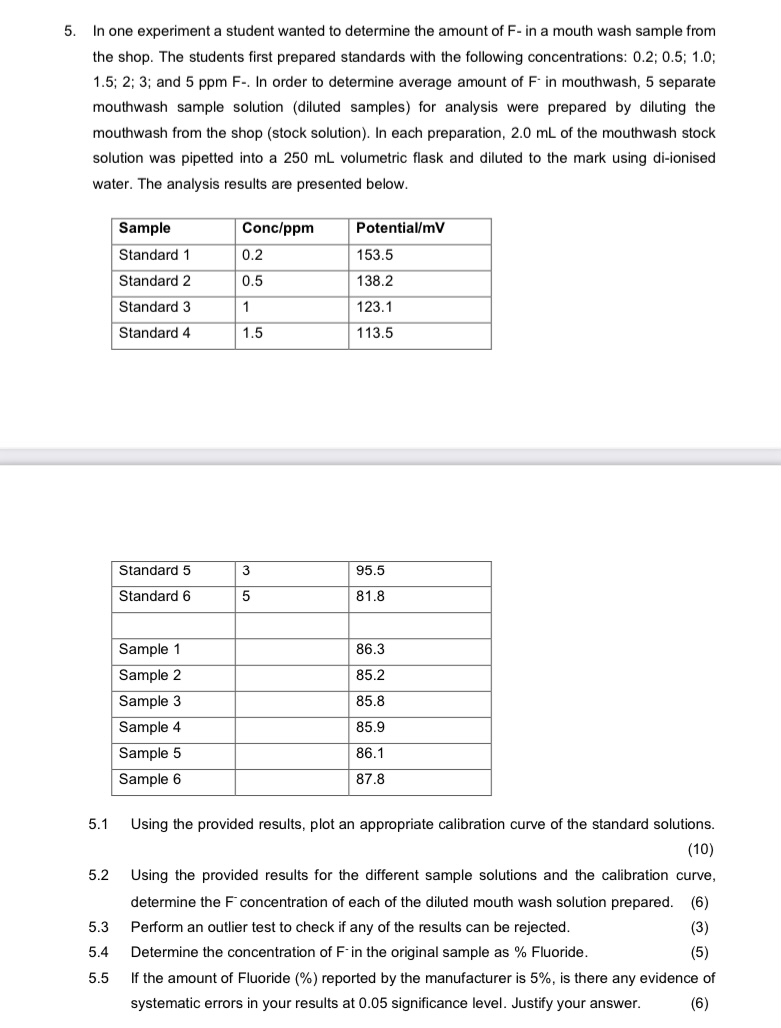
5. In one experiment a student wanted to determine the amount of F- in a mouth wash sample from the shop. The students first prepared standards with the following concentrations: 0.2; 0.5; 1.0; 1.5; 2; 3; and 5 ppm F-. In order to determine average amount of F in mouthwash, 5 separate mouthwash sample solution (diluted samples) for analysis were prepared by diluting the mouthwash from the shop (stock solution). In each preparation, 2.0 mL of the mouthwash stock solution was pipetted into a 250 mL volumetric flask and diluted to the mark using di-ionised water. The analysis results are presented below. Sample Conc/ppm Potential/mV Standard 1 0.2 153.5 Standard 2 0.5 138.2 Standard 3 1 123.1 Standard 4 1.5 113.5 Standard 5 3 95.5 Standard 6 5 81.8 Sample 1 86.3 Sample 2 85.2 Sample 3 85.8 Sample 4 85.9 Sample 5 86.1 Sample 6 87.8 5.1 5.3 5.4 Using the provided results, plot an appropriate calibration curve of the standard solutions. (10) 5.2 Using the provided results for the different sample solutions and the calibration curve, determine the F concentration of each of the diluted mouth wash solution prepared. (6) Perform an outlier test to check if any of the results can be rejected. Determine the concentration of F-in the original sample as % Fluoride. (3) (5) 5.5 If the amount of Fluoride (%) reported by the manufacturer is 5%, is there any evidence of systematic errors in your results at 0.05 significance level. Justify your answer. (6)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


