Answered step by step
Verified Expert Solution
Question
1 Approved Answer
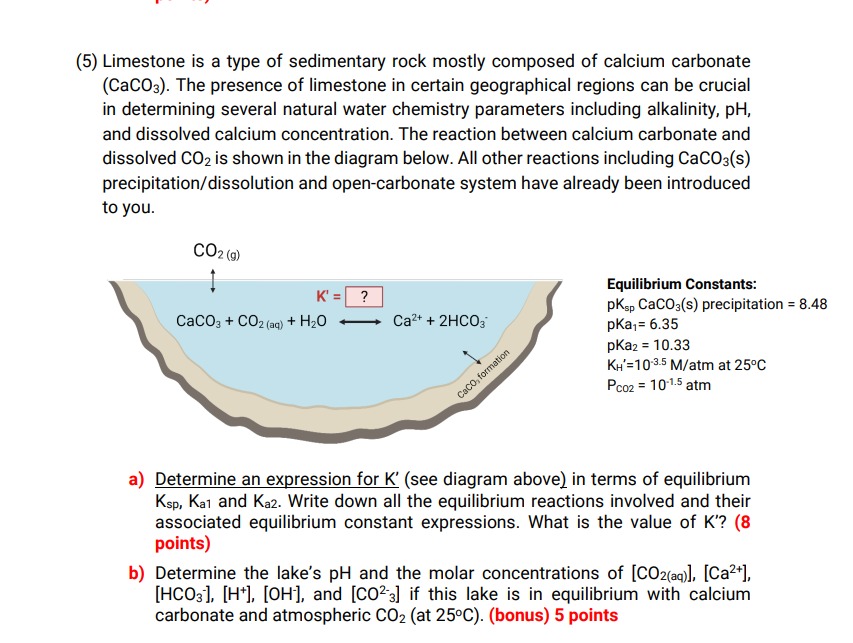
( 5 ) Limestone is a type of sedimentary rock mostly composed of calcium carbonate ( C a C O 3 ) . The presence
Limestone is a type of sedimentary rock mostly composed of calcium carbonate
The presence of limestone in certain geographical regions can be crucial
in determining several natural water chemistry parameters including alkalinity,
and dissolved calcium concentration. The reaction between calcium carbonate and
dissolved is shown in the diagram below. All other reactions including s
precipitationdissolution and opencarbonate system have already been introduced
to you.
Equilibrium Constants:
precipitation
atm
a Determine an expression for see diagram above in terms of equilibrium
and Write down all the equilibrium reactions involved and their
associated equilibrium constant expressions. What is the value of
points
b Determine the lake's and the molar concentrations of
and if this lake is in equilibrium with calcium
carbonate and atmospheric at bonus points
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


