Answered step by step
Verified Expert Solution
Question
1 Approved Answer
5. Methylene chloride (species A ) undergoes an interphase convective mass-transfer process between air and water at 20C and 1.80 atm total pressure. Air is
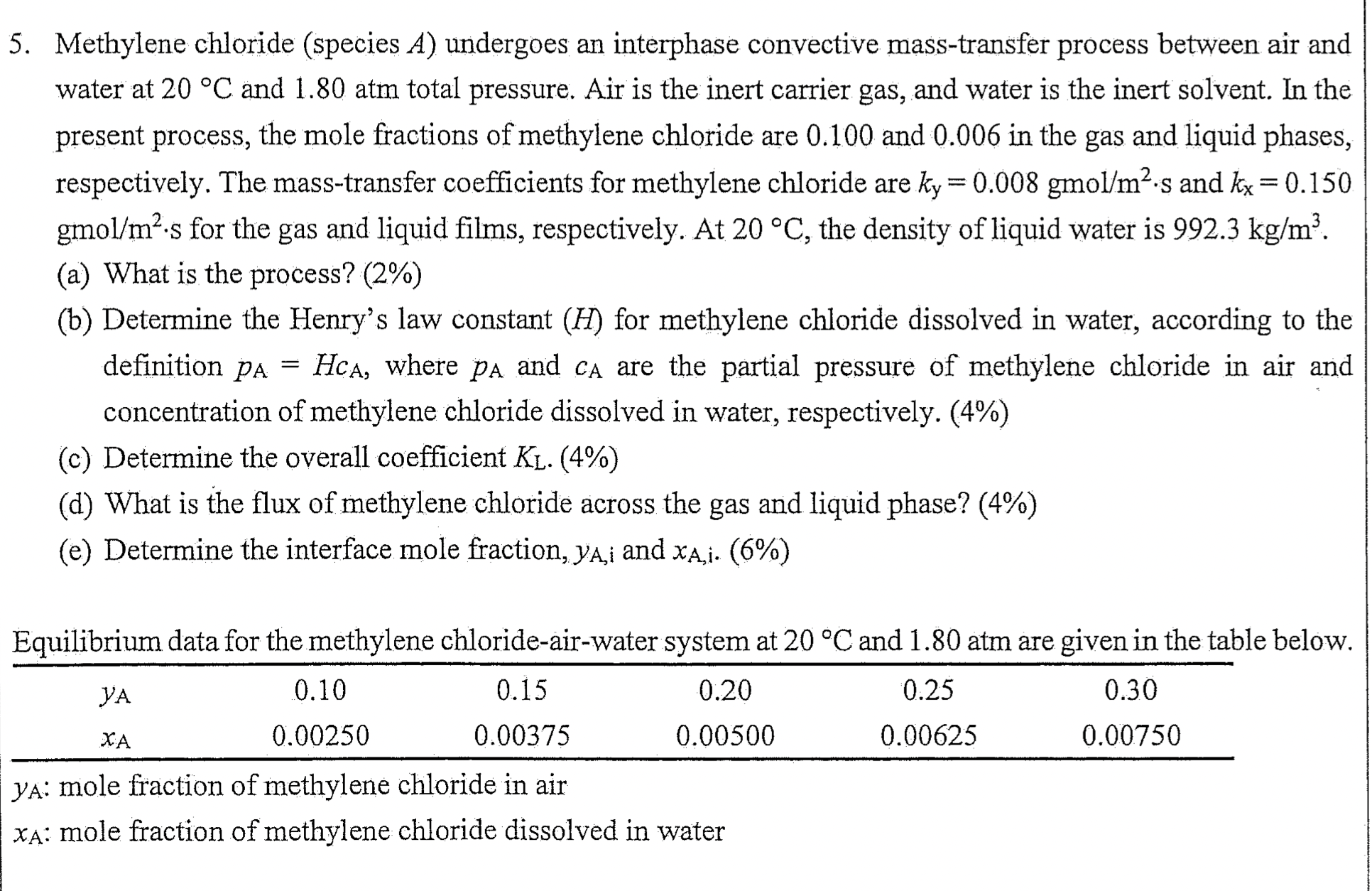 5. Methylene chloride (species A ) undergoes an interphase convective mass-transfer process between air and water at 20C and 1.80 atm total pressure. Air is the inert carrier gas, and water is the inert solvent. In the present process, the mole fractions of methylene chloride are 0.100 and 0.006 in the gas and liquid phases, respectively. The mass-transfer coefficients for methylene chloride are ky=0.008gmol/m2s and kx=0.150 gmol /m2s for the gas and liquid films, respectively. At 20C, the density of liquid water is 992.3kg/m3. (a) What is the process? (2\%) (b) Determine the Henry's law constant (H) for methylene chloride dissolved in water, according to the definition pA=HcA, where pA and cA are the partial pressure of methylene chloride in air and concentration of methylene chloride dissolved in water, respectively. (4\%) (c) Determine the overall coefficient KL. (4\%) (d) What is the flux of methylene chloride across the gas and liquid phase? (4\%) (e) Determine the interface mole fraction, yA,i and xA,i. (6%) Equilibrium data for the methylene chloride-air-water system at 20C and 1.80 atm are given in the table below. yA : mole fraction of methylene chloride in air xA : mole fraction of methylene chloride dissolved in water 5. Methylene chloride (species A ) undergoes an interphase convective mass-transfer process between air and water at 20C and 1.80 atm total pressure. Air is the inert carrier gas, and water is the inert solvent. In the present process, the mole fractions of methylene chloride are 0.100 and 0.006 in the gas and liquid phases, respectively. The mass-transfer coefficients for methylene chloride are ky=0.008gmol/m2s and kx=0.150 gmol /m2s for the gas and liquid films, respectively. At 20C, the density of liquid water is 992.3kg/m3. (a) What is the process? (2\%) (b) Determine the Henry's law constant (H) for methylene chloride dissolved in water, according to the definition pA=HcA, where pA and cA are the partial pressure of methylene chloride in air and concentration of methylene chloride dissolved in water, respectively. (4\%) (c) Determine the overall coefficient KL. (4\%) (d) What is the flux of methylene chloride across the gas and liquid phase? (4\%) (e) Determine the interface mole fraction, yA,i and xA,i. (6%) Equilibrium data for the methylene chloride-air-water system at 20C and 1.80 atm are given in the table below. yA : mole fraction of methylene chloride in air xA : mole fraction of methylene chloride dissolved in water
5. Methylene chloride (species A ) undergoes an interphase convective mass-transfer process between air and water at 20C and 1.80 atm total pressure. Air is the inert carrier gas, and water is the inert solvent. In the present process, the mole fractions of methylene chloride are 0.100 and 0.006 in the gas and liquid phases, respectively. The mass-transfer coefficients for methylene chloride are ky=0.008gmol/m2s and kx=0.150 gmol /m2s for the gas and liquid films, respectively. At 20C, the density of liquid water is 992.3kg/m3. (a) What is the process? (2\%) (b) Determine the Henry's law constant (H) for methylene chloride dissolved in water, according to the definition pA=HcA, where pA and cA are the partial pressure of methylene chloride in air and concentration of methylene chloride dissolved in water, respectively. (4\%) (c) Determine the overall coefficient KL. (4\%) (d) What is the flux of methylene chloride across the gas and liquid phase? (4\%) (e) Determine the interface mole fraction, yA,i and xA,i. (6%) Equilibrium data for the methylene chloride-air-water system at 20C and 1.80 atm are given in the table below. yA : mole fraction of methylene chloride in air xA : mole fraction of methylene chloride dissolved in water 5. Methylene chloride (species A ) undergoes an interphase convective mass-transfer process between air and water at 20C and 1.80 atm total pressure. Air is the inert carrier gas, and water is the inert solvent. In the present process, the mole fractions of methylene chloride are 0.100 and 0.006 in the gas and liquid phases, respectively. The mass-transfer coefficients for methylene chloride are ky=0.008gmol/m2s and kx=0.150 gmol /m2s for the gas and liquid films, respectively. At 20C, the density of liquid water is 992.3kg/m3. (a) What is the process? (2\%) (b) Determine the Henry's law constant (H) for methylene chloride dissolved in water, according to the definition pA=HcA, where pA and cA are the partial pressure of methylene chloride in air and concentration of methylene chloride dissolved in water, respectively. (4\%) (c) Determine the overall coefficient KL. (4\%) (d) What is the flux of methylene chloride across the gas and liquid phase? (4\%) (e) Determine the interface mole fraction, yA,i and xA,i. (6%) Equilibrium data for the methylene chloride-air-water system at 20C and 1.80 atm are given in the table below. yA : mole fraction of methylene chloride in air xA : mole fraction of methylene chloride dissolved in water Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


