Answered step by step
Verified Expert Solution
Question
1 Approved Answer
5. One mole ideal monoatomic gas is heated in two different processes according to path AB and AC. If temperature of state B and
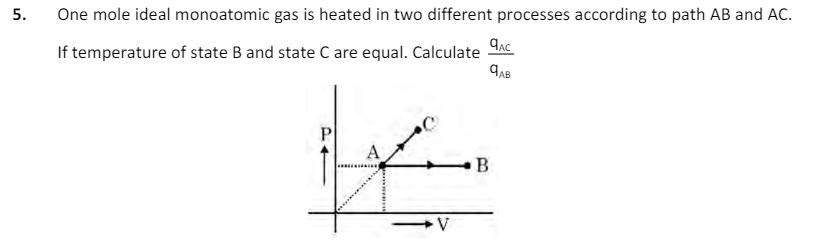
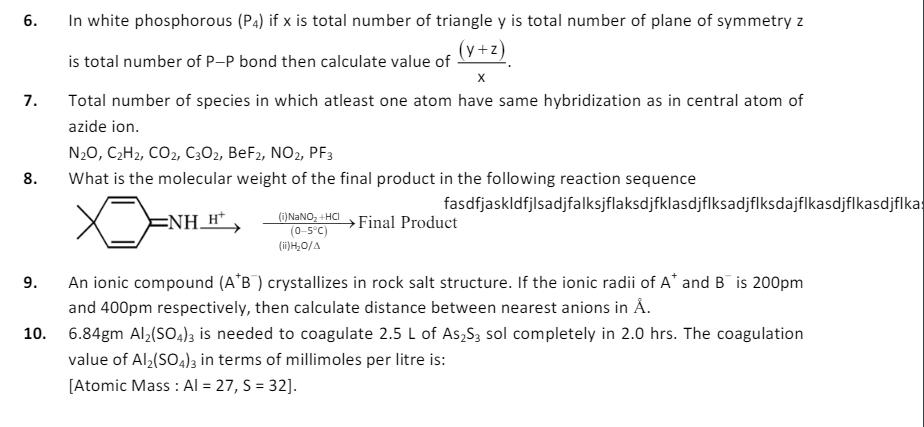
5. One mole ideal monoatomic gas is heated in two different processes according to path AB and AC. If temperature of state B and state C are equal. Calculate P B 6. 7. 8. 9. In white phosphorous (P4) if x is total number of triangle y is total number of plane of symmetry z (y+z) is total number of P-P bond then calculate value of X Total number of species in which atleast one atom have same hybridization as in central atom of azide ion. N2O, CH2, CO2, C302, BeF2, NO2, PF3 What is the molecular weight of the final product in the following reaction sequence NH_H (i) NaNO+HCI (0-5C) (ii) HO/A fasdfjaskldfjlsadjfalksjflaksdjfklasdjflksadjflksdajflkasdjflkasdjflka Final Product An ionic compound (A+B) crystallizes in rock salt structure. If the ionic radii of A and B is 200pm and 400pm respectively, then calculate distance between nearest anions in . 10. 6.84gm Al2(SO4)3 is needed to coagulate 2.5 L of AsS3 sol completely in 2.0 hrs. The coagulation value of Al2(SO4)3 in terms of millimoles per litre is: [Atomic Mass: Al = 27, S = 32].
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


