Question
5. Phosphate ions are abundant in cells, both as the ions themselves and as important sub- stituents on organic molecules. Most importantly, the pK,
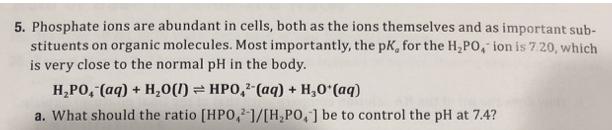
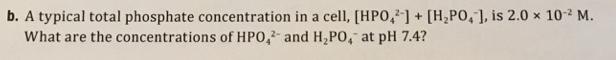
5. Phosphate ions are abundant in cells, both as the ions themselves and as important sub- stituents on organic molecules. Most importantly, the pK, for the H,PO, ion is 7 20, which is very close to the normal pH in the body. H,PO, (aq) + H,0(1) = HPO, (aq) + H,0 (aq) a. What should the ratio [HPO,1/[H,PO,] be to control the pH at 7.4? b. A typical total phosphate concentration in a cell, [HPO,]+ [H,PO, ], is 2.0 x 10- M. What are the concentrations of HPO, and H,PO, at pH 7.4? 2-
Step by Step Solution
3.29 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Organic Chemistry
Authors: L. G. Wade Jr.
8th edition
321768418, 978-0321768414
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



