Question
Gallium sulfide is a compound that is used as a semiconductor in electronics. It is synthesized from elemental gallium and sulfur, according to the
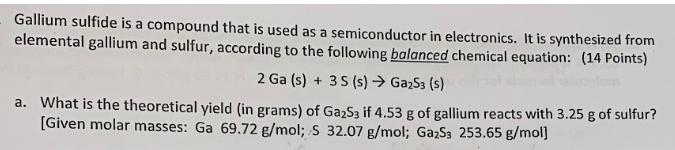
Gallium sulfide is a compound that is used as a semiconductor in electronics. It is synthesized from elemental gallium and sulfur, according to the following balanced chemical equation: (14 Points) 2 Ga (s)3 S (s) Ga2S3 (s) What is the theoretical yield (in grams) of Ga2S3 if 4.53 g of gallium reacts with 3.25 g of sulfur? [Given molar masses: Ga 69.72 g/mol; S 32.07 g/mol; Ga2Ss 253.65 g/mol a.
Step by Step Solution
3.37 Rating (147 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Essentials of Economics
Authors: Bradley Schiller, Karen Gebhardt
10th edition
125923570X, 978-1259235702
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



