Answered step by step
Verified Expert Solution
Question
1 Approved Answer
. (5 points) A system consisting of air in a piston-cylinder initially at 300 F and 1 bar experiences different types of interactions as
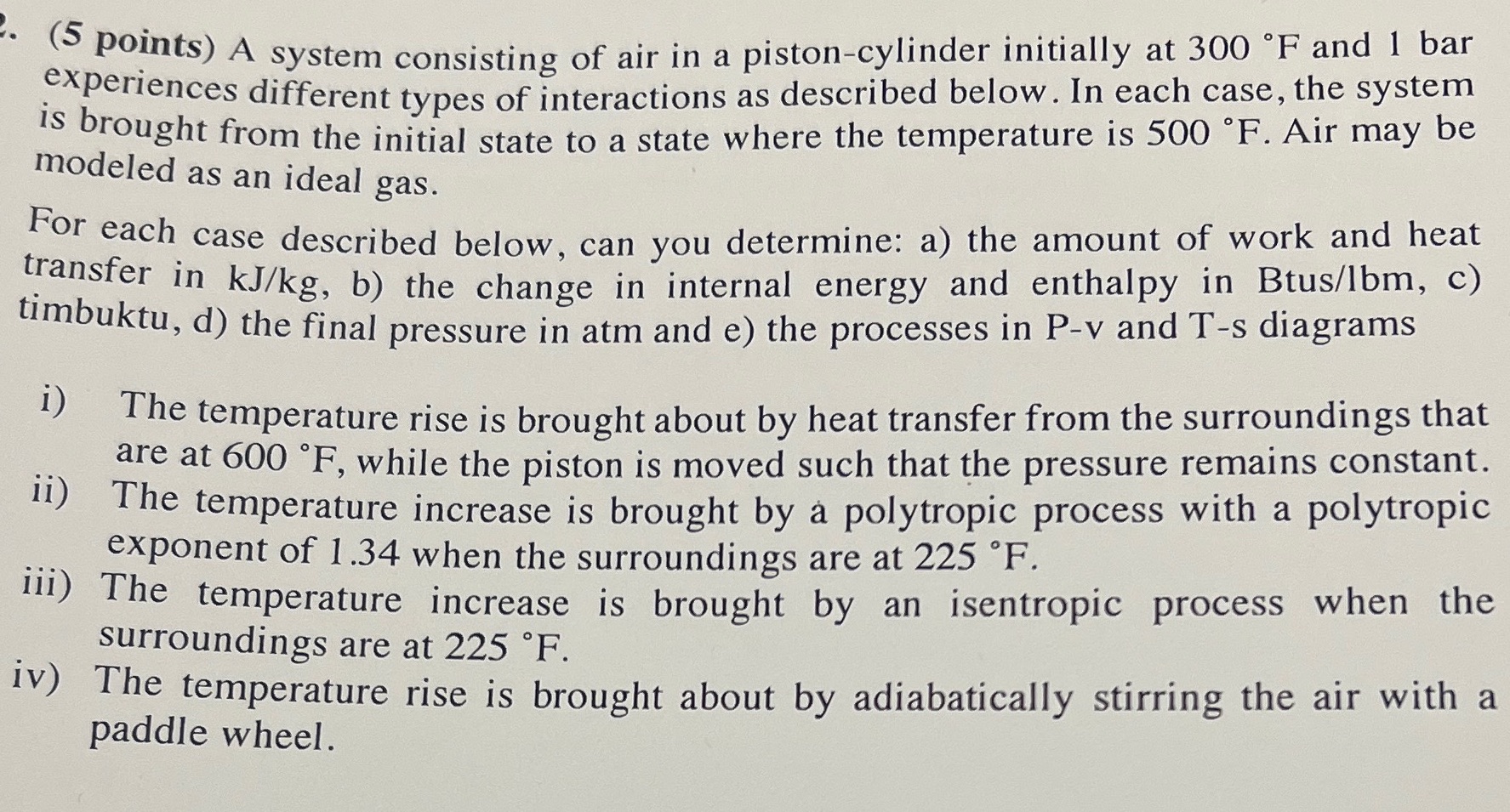
. (5 points) A system consisting of air in a piston-cylinder initially at 300 F and 1 bar experiences different types of interactions as described below. In each case, the system is brought from the initial state to a state where the temperature is 500 F. Air may be modeled as an ideal gas. For each case described below, can you determine: a) the amount of work and heat transfer in kJ/kg, b) the change in internal energy and enthalpy in Btus/lbm, c) timbuktu, d) the final pressure in atm and e) the processes in P-v and T-s diagrams i) ii) The temperature rise is brought about by heat transfer from the surroundings that are at 600 F, while the piston is moved such that the pressure remains constant. The temperature increase is brought by a polytropic process with a polytropic exponent of 1.34 when the surroundings are at 225 F. iii) The temperature increase is brought by an isentropic process when the surroundings are at 225 F. iv) The temperature rise is brought about by adiabatically stirring the air with a paddle wheel.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
case i Heat transfer at constant pressure a Since the process is at constant pressure the work done can be calculated as W PV and the heat transfer ca...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


