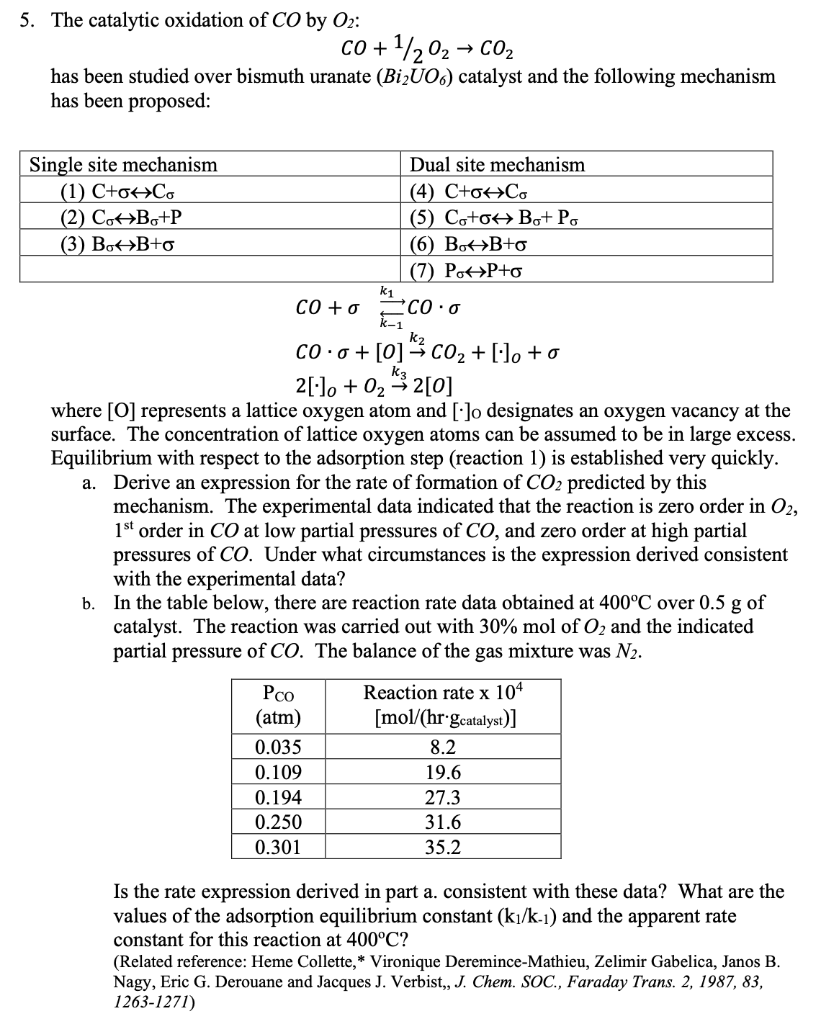
5. The catalytic oxidation of CO by 02: co +1/202 + CO2 has been studied over bismuth uranate (Bi2006) catalyst and the following mechanism has been proposed: Single site mechanism Dual site mechanism (1) C+ohco (4) C+OHC (2) CoHBo+P (5) Co+OHBo+ Po (3) BoHB+o (6) BotB+o (7) PotP+o k1 CO +o *CO.0 K-1 00.0 +[0] ** C02 + [-1. +o k3 2[-1. + 02 -32[0] where [O] represents a lattice oxygen atom and [.]o designates an oxygen vacancy at the surface. The concentration of lattice oxygen atoms can be assumed to be in large excess. Equilibrium with respect to the adsorption step (reaction 1) is established very quickly. a. Derive an expression for the rate of formation of CO2 predicted by this mechanism. The experimental data indicated that the reaction is zero order in 02, 1st order in CO at low partial pressures of CO, and zero order at high partial pressures of CO. Under what circumstances is the expression derived consistent with the experimental data? b. In the table below, there are reaction rate data obtained at 400C over 0.5 g of catalyst. The reaction was carried out with 30% mol of O2 and the indicated partial pressure of CO. The balance of the gas mixture was N2. Pco (atm) 0.035 0.109 0.194 0.250 0.301 Reaction rate x 104 [mol/(hr-gcatalyst)] 8.2 19.6 27.3 31.6 35.2 Is the rate expression derived in part a. consistent with these data? What are the values of the adsorption equilibrium constant (kJ/k-1) and the apparent rate constant for this reaction at 400C? (Related reference: Heme Collette, * Vironique Deremince-Mathieu, Zelimir Gabelica, Janos B. Nagy, Eric G. Derouane and Jacques J. Verbist,, J. Chem. SOC., Faraday Trans. 2, 1987, 83, 1263-1271) 5. The catalytic oxidation of CO by 02: co +1/202 + CO2 has been studied over bismuth uranate (Bi2006) catalyst and the following mechanism has been proposed: Single site mechanism Dual site mechanism (1) C+ohco (4) C+OHC (2) CoHBo+P (5) Co+OHBo+ Po (3) BoHB+o (6) BotB+o (7) PotP+o k1 CO +o *CO.0 K-1 00.0 +[0] ** C02 + [-1. +o k3 2[-1. + 02 -32[0] where [O] represents a lattice oxygen atom and [.]o designates an oxygen vacancy at the surface. The concentration of lattice oxygen atoms can be assumed to be in large excess. Equilibrium with respect to the adsorption step (reaction 1) is established very quickly. a. Derive an expression for the rate of formation of CO2 predicted by this mechanism. The experimental data indicated that the reaction is zero order in 02, 1st order in CO at low partial pressures of CO, and zero order at high partial pressures of CO. Under what circumstances is the expression derived consistent with the experimental data? b. In the table below, there are reaction rate data obtained at 400C over 0.5 g of catalyst. The reaction was carried out with 30% mol of O2 and the indicated partial pressure of CO. The balance of the gas mixture was N2. Pco (atm) 0.035 0.109 0.194 0.250 0.301 Reaction rate x 104 [mol/(hr-gcatalyst)] 8.2 19.6 27.3 31.6 35.2 Is the rate expression derived in part a. consistent with these data? What are the values of the adsorption equilibrium constant (kJ/k-1) and the apparent rate constant for this reaction at 400C? (Related reference: Heme Collette, * Vironique Deremince-Mathieu, Zelimir Gabelica, Janos B. Nagy, Eric G. Derouane and Jacques J. Verbist,, J. Chem. SOC., Faraday Trans. 2, 1987, 83, 1263-1271)







