Answered step by step
Verified Expert Solution
Question
1 Approved Answer
5. The P.E. diagram below shows an uncatalyzed exothermic reaction (solid line) and a corresponding catalyzed reaction (dashed line) (a) What is the activation energy
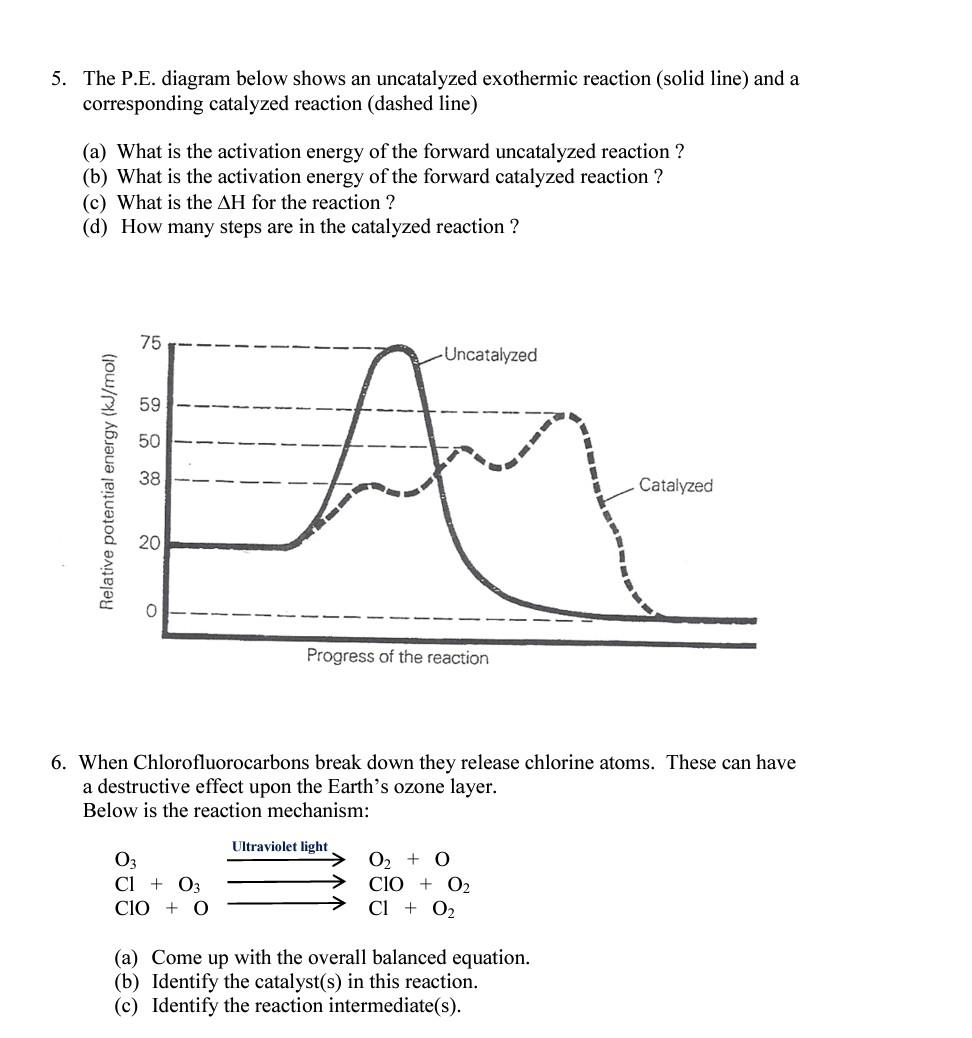
5. The P.E. diagram below shows an uncatalyzed exothermic reaction (solid line) and a corresponding catalyzed reaction (dashed line) (a) What is the activation energy of the forward uncatalyzed reaction? (b) What is the activation energy of the forward catalyzed reaction? (c) What is the H for the reaction? (d) How many steps are in the catalyzed reaction? 6. When Chlorofluorocarbons break down they release chlorine atoms. These can have a destructive effect upon the Earth's ozone layer. Below is the reaction mechanism: (a) Come up with the overall balanced equation. (b) Identify the catalyst(s) in this reaction. (c) Identify the reaction intermediate(s)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


