Answered step by step
Verified Expert Solution
Question
1 Approved Answer
50 mg of benzil 10mg of NABH4 0.5 ml of ethanol water 0.5 ml experiment is synthesis of hydrobenzoin using reduction please give rhe theoretical
50 mg of benzil 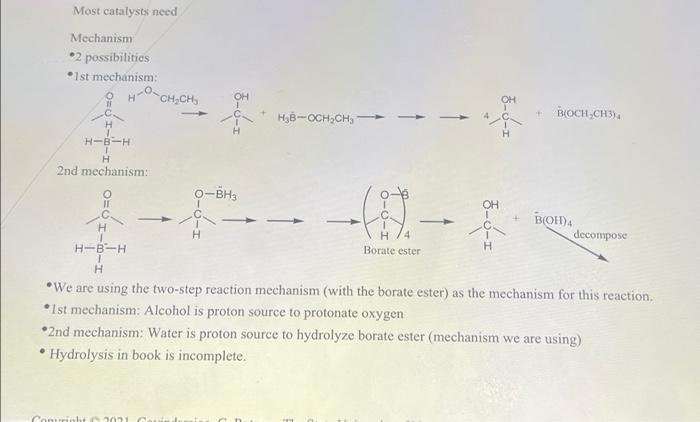
Most catalysts need Mechanism *2 possibilities 1st mechanism: CH,CH, H OH OH HB-OCH.CH 3-6-3 BOCH,CH3)4 H H-BAH H 2nd mechanism: O-BHs OH II H C -(I). - BOH) decompose H-B-H H/4 Borate ester H H We are using the two-step reaction mechanism (with the borate ester) as the mechanism for this reaction, *Ist mechanism: Alcohol is proton source to protonate oxygen 2nd mechanism: Water is proton source to hydrolyze borate ester (mechanism we are using) Hydrolysis in book is incomplete. Cash 20 10mg of NABH4
0.5 ml of ethanol
water 0.5 ml
experiment is synthesis of hydrobenzoin using reduction
please give rhe theoretical yield of hydrobenzoin in the reaction. thx
pls show how to determine the theoretical yield

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


