Answered step by step
Verified Expert Solution
Question
1 Approved Answer
5.2 hw 10 questions QUESTION 1 Lewis theory predicts that the formula for a compound between fluorine and calcium is: O A. CaF OB. CaF2
5.2 hw 10 questions 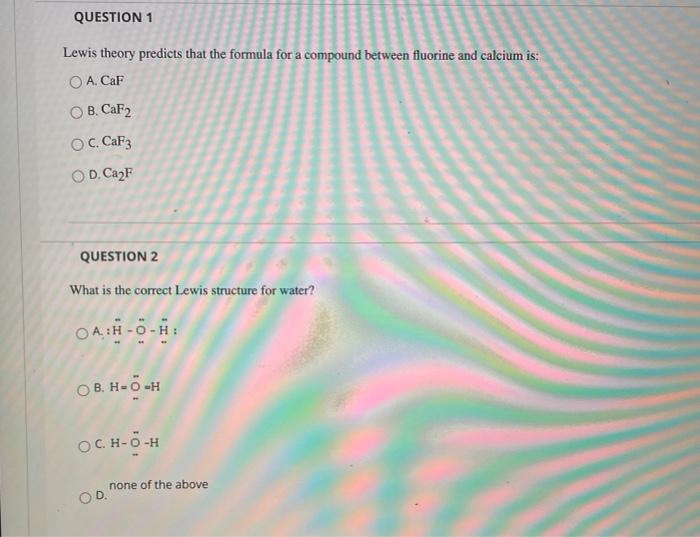

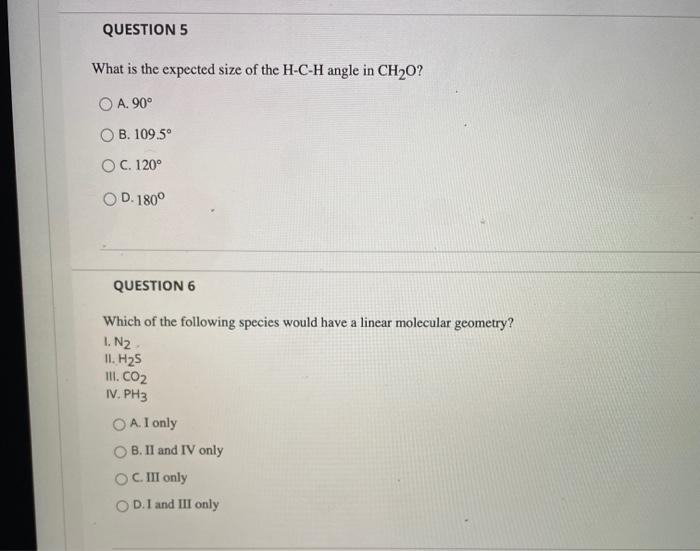
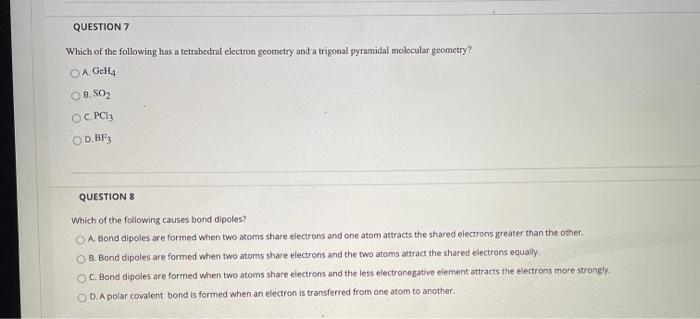

QUESTION 1 Lewis theory predicts that the formula for a compound between fluorine and calcium is: O A. CaF OB. CaF2 . CaF3 OD. Ca2F QUESTION 2 What is the correct Lewis structure for water? OA:H-- OB. H-7-H . -- none of the above QUESTION 3 The correct Lewis structure for BF3 would have exactly: A. no double bonds. B. 1 double bond. OC. two double bonds. OD. one triple bond. QUESTION 4 What is the angle between electron groups in the trigonal planar electron geometry? O A. 109.5 OB. 120 O C. 180 D.not enough information to be able to tell QUESTION 5 What is the expected size of the H-C-H angle in CH20? A. 90 OB. 109.5 O C. 120 OD. 180 QUESTION 6 Which of the following species would have a linear molecular geometry? I.N2 II. H25 III. CO2 IV. PH3 O A. I only B. II and IV only OC. III only OD. I and III only QUESTION 7 Which of the following has a tetrahedral electron geometry and a trigonal pyramidal molecular geometry? O A GcHQ OB. SO2 OC PCIE OD.BF QUESTIONS Which of the following causes bond dipoles? O A Bond dipoles are formed when two atoms share electrons and one atom attracts the shared electrons greater than the other B. Bond dipoles are formed when two atoms share electrons and the two atoms attract the shared electrons equally OC. Bond dipoles are formed when two atoms share electrons and the less electronegative element attracts the electrons more strongly OD. A polar covalent bond is formed when an electron is transferred from one atom to another. QUESTION 9 Which molecule listed below is a polar molecule? O A. H20 B. HCN O C. NF3 OD. all of the above QUESTION 10 Which of the following molecules have polar bonds but is nonpolar? OA. CO2 OB. BF3 OC. CC14 D. all of the above 




Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


