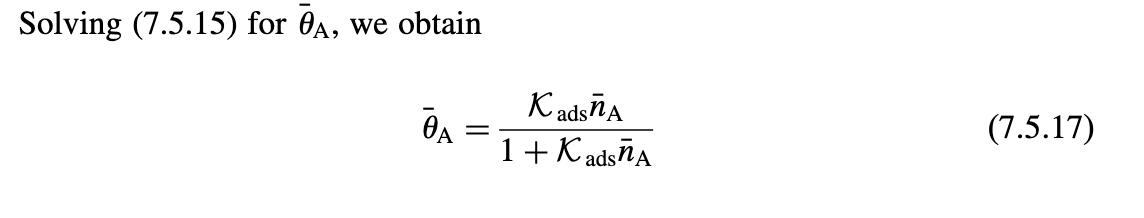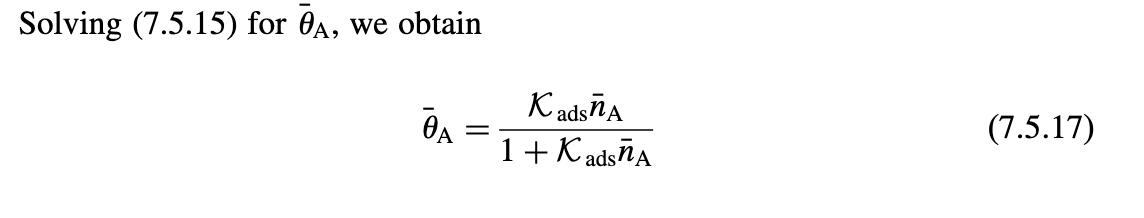
5.3. Surface Model for Silicon Etch in a CF4 Discharge Consider the following surface model for pure chemical silicon etch (no ion bombardment) in a CF4 discharge. Let n1 and n2 be the gas-phase densities of CFx radicals and F atoms near the surface, respectively, and let 1 and 2 be the fractions of the SiF3 surface covered with CF4 and SiF4, respectively. Let Ka1 and Ka (cm3/s) be the adsorption rate constants for CFx radicals and F atoms, respectively, and let Kd1 and Kd2 be the desorption rate constants (s1) for CF4(g) and SiF4(g), respectively. Assume Langmuir kinetics with adsorption of CFx and F on the SiF3 surface only. PROBLEMS 617 (a) In the steady state, give the two conservation equations for carbon and fluorine on the surface. (b) Solve these to obtain the surfaces coverages 1 and 2. (c) Find the silicon etch rate ESi and plot the normalized etch rate per incident F atom, ESi2(AAm3/min) versus n21 for x=3,Ka1=Ka2= 41014cm3/s,n0=71014cm2,Kd1=Kd2=1012s1, and nSiF3=51022cm3. Assume that n1,n2Kd2/Ka1. Solving (7.5.15) for A, we obtain A=1+KadsnAKadsnA 5.3. Surface Model for Silicon Etch in a CF4 Discharge Consider the following surface model for pure chemical silicon etch (no ion bombardment) in a CF4 discharge. Let n1 and n2 be the gas-phase densities of CFx radicals and F atoms near the surface, respectively, and let 1 and 2 be the fractions of the SiF3 surface covered with CF4 and SiF4, respectively. Let Ka1 and Ka (cm3/s) be the adsorption rate constants for CFx radicals and F atoms, respectively, and let Kd1 and Kd2 be the desorption rate constants (s1) for CF4(g) and SiF4(g), respectively. Assume Langmuir kinetics with adsorption of CFx and F on the SiF3 surface only. PROBLEMS 617 (a) In the steady state, give the two conservation equations for carbon and fluorine on the surface. (b) Solve these to obtain the surfaces coverages 1 and 2. (c) Find the silicon etch rate ESi and plot the normalized etch rate per incident F atom, ESi2(AAm3/min) versus n21 for x=3,Ka1=Ka2= 41014cm3/s,n0=71014cm2,Kd1=Kd2=1012s1, and nSiF3=51022cm3. Assume that n1,n2Kd2/Ka1. Solving (7.5.15) for A, we obtain A=1+KadsnAKadsnA