Answered step by step
Verified Expert Solution
Question
1 Approved Answer
56. If the maximum concentration of PbCl2 in water is 0.01M at 298 K. Its maximum concentration in 0.1M NaCl will be : (1)

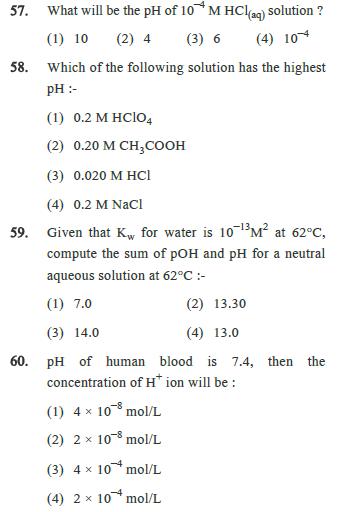
56. If the maximum concentration of PbCl2 in water is 0.01M at 298 K. Its maximum concentration in 0.1M NaCl will be : (1) 4 103 M x (3) 4 102 M (2) 0.4 10M x (4) 410 M 57. What will be the pH of 10* M HCl(aq) solution? (1) 10 (2) 4 (3) 6 (4) 104 58. Which of the following solution has the highest pH:- (1) 0.2 M HClO4 (2) 0.20 M CH3COOH (3) 0.020 M HCl (4) 0.2 M NaCl 59. Given that Kw for water is 10 13 M at 62C, compute the sum of pOH and pH for a neutral aqueous solution at 62C :- (1) 7.0 (3) 14.0 (2) 13.30 (4) 13.0 60. pH of human blood is 7.4, then the concentration of H+ ion will be: (1) 4 10 mol/L (2) 2 103 mol/L (3) 410 mol/L (4) 2x10 mol/L
Step by Step Solution
There are 3 Steps involved in it
Step: 1
56 The maximum concentration of PbCl2 in 01M NaCl can be calculated using the common ion effect The ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


