Answered step by step
Verified Expert Solution
Question
1 Approved Answer
5-8. Consider two different, immobilized atoms, A and B, with only two thermally accessible energy modes: an electronic excited state and a magnetic 'spin' state.

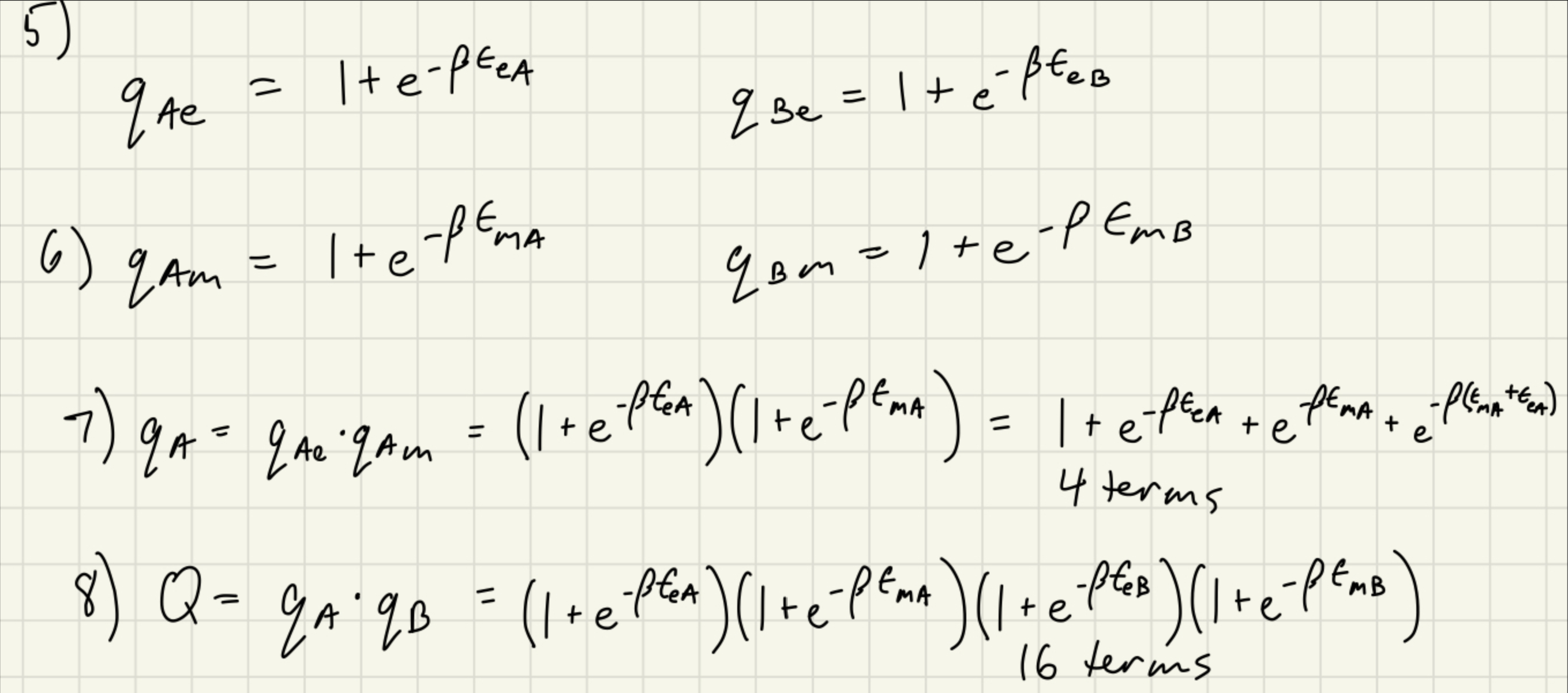 5-8. Consider two different, immobilized atoms, A and B, with only two thermally accessible energy modes: an electronic excited state and a magnetic 'spin' state. Due to them being immobilized, there is no translation energy to consider, and also the molecules are distinguishable. The energy of the electronic excited state for molecule A is eA, while that for molecule B, is eB. The magnetic spin state has zero energy when the spin is 'down' on either molecule and mA when the spin is 'up' on molecule A and mB when the spin is 'up' on molecule B. 5) What are the molecular (i.e. microcanonical) partition functions, qAe and qBe for the electronic mode for molecules A and B ? 6) What are the molecular (i.e. microcanonical) partition functions, qAm and qBm for the magnetic spin mode for molecules A and B ? 7) Fort the partition functions in 5 and 6, you should have two terms. Consider the total energy of an A atom with both electronic and magnetic modes. How many terms are there in the total molecular partition function? 8) Now consider a system consisting of one atom of A and one of atom B. How many terms are there in the system partition function? 5) qAe=1+eeAqBe=1+eeB 6) qAm=1+emAqBm=1+emB 7) qA=qAeqAm=(1+eeA)(1+emA)=1+eEA+eMA+emA+E 4 terms 8) Q=qAqB=(1+eEA)(1+eMA)(1+eB)(1+eMB) 16 terms
5-8. Consider two different, immobilized atoms, A and B, with only two thermally accessible energy modes: an electronic excited state and a magnetic 'spin' state. Due to them being immobilized, there is no translation energy to consider, and also the molecules are distinguishable. The energy of the electronic excited state for molecule A is eA, while that for molecule B, is eB. The magnetic spin state has zero energy when the spin is 'down' on either molecule and mA when the spin is 'up' on molecule A and mB when the spin is 'up' on molecule B. 5) What are the molecular (i.e. microcanonical) partition functions, qAe and qBe for the electronic mode for molecules A and B ? 6) What are the molecular (i.e. microcanonical) partition functions, qAm and qBm for the magnetic spin mode for molecules A and B ? 7) Fort the partition functions in 5 and 6, you should have two terms. Consider the total energy of an A atom with both electronic and magnetic modes. How many terms are there in the total molecular partition function? 8) Now consider a system consisting of one atom of A and one of atom B. How many terms are there in the system partition function? 5) qAe=1+eeAqBe=1+eeB 6) qAm=1+emAqBm=1+emB 7) qA=qAeqAm=(1+eeA)(1+emA)=1+eEA+eMA+emA+E 4 terms 8) Q=qAqB=(1+eEA)(1+eMA)(1+eB)(1+eMB) 16 terms Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


