Answered step by step
Verified Expert Solution
Question
1 Approved Answer
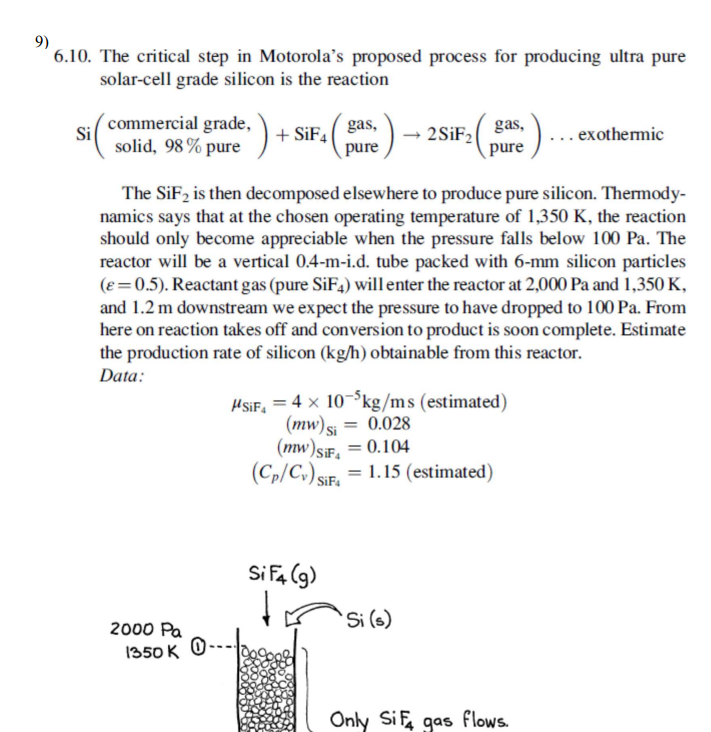
6 . 1 0 . The critical step in Motorola's proposed process for producing ultra pure solar - cell grade silicon is the reaction S
The critical step in Motorola's proposed process for producing ultra pure
solarcell grade silicon is the reaction
commercial grade, solid, pure gas, pure gas, pure exothermic
The is then decomposed elsewhere to produce pure silicon. Thermody
namics says that at the chosen operating temperature of the reaction
should only become appreciable when the pressure falls below The
reactor will be a vertical d tube packed with silicon particles
Reactant gas pure will enter the reactor at and
and downstream we expect the pressure to have dropped to From
here on reaction takes off and conversion to product is soon complete. Estimate
the production rate of silicon obtainable from this reactor.
Data:
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


