Answered step by step
Verified Expert Solution
Question
1 Approved Answer
6. For which of following reversible reaction the degree of dissociation or association depends upon the value of temperature but NOT on value of
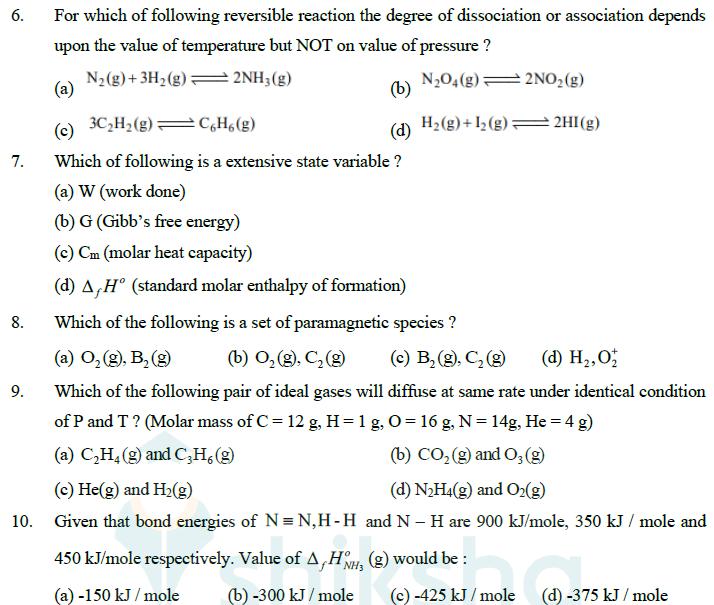
6. For which of following reversible reaction the degree of dissociation or association depends upon the value of temperature but NOT on value of pressure? (a) N2(g)+3H2(g)2NH3(g) (b) N2O4(g) 2NO2(g) (c) 3C2H2(g) C6H6(g) (d) H2(g)+12(g): 2HI(g) 7. Which of following is a extensive state variable? (a) W (work done) (b) G (Gibb's free energy) (c) Cm (molar heat capacity) (d) A,H (standard molar enthalpy of formation) Which of the following is a set of paramagnetic species ? 8. (a) O (g), B (g) 9. (b) O2(g), C(g) (c) B(g), C (g) (d) H,O Which of the following pair of ideal gases will diffuse at same rate under identical condition of P and T? (Molar mass of C = 12 g, H = 1 g, O= 16 g, N = 14g, He = 4 g) (a) C2H4(g) and C3H6 (g) (c) He(g) and H2(g) (b) CO2 (g) and O3(g) (d) N2H4(g) and O2(g) 10. Given that bond energies of N = N,H-H and NH are 900 kJ/mole, 350 kJ/mole and 450 kJ/mole respectively. Value of A,HH3 (g) would be: (a) -150 kJ/mole (b) -300 kJ/mole (c) -425 kJ/mole (d) -375 kJ/mole
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


