Answered step by step
Verified Expert Solution
Question
1 Approved Answer
6. In the class, we have gone through the example of polyenes in the context of the 1. D PIB model. Now, let's work on
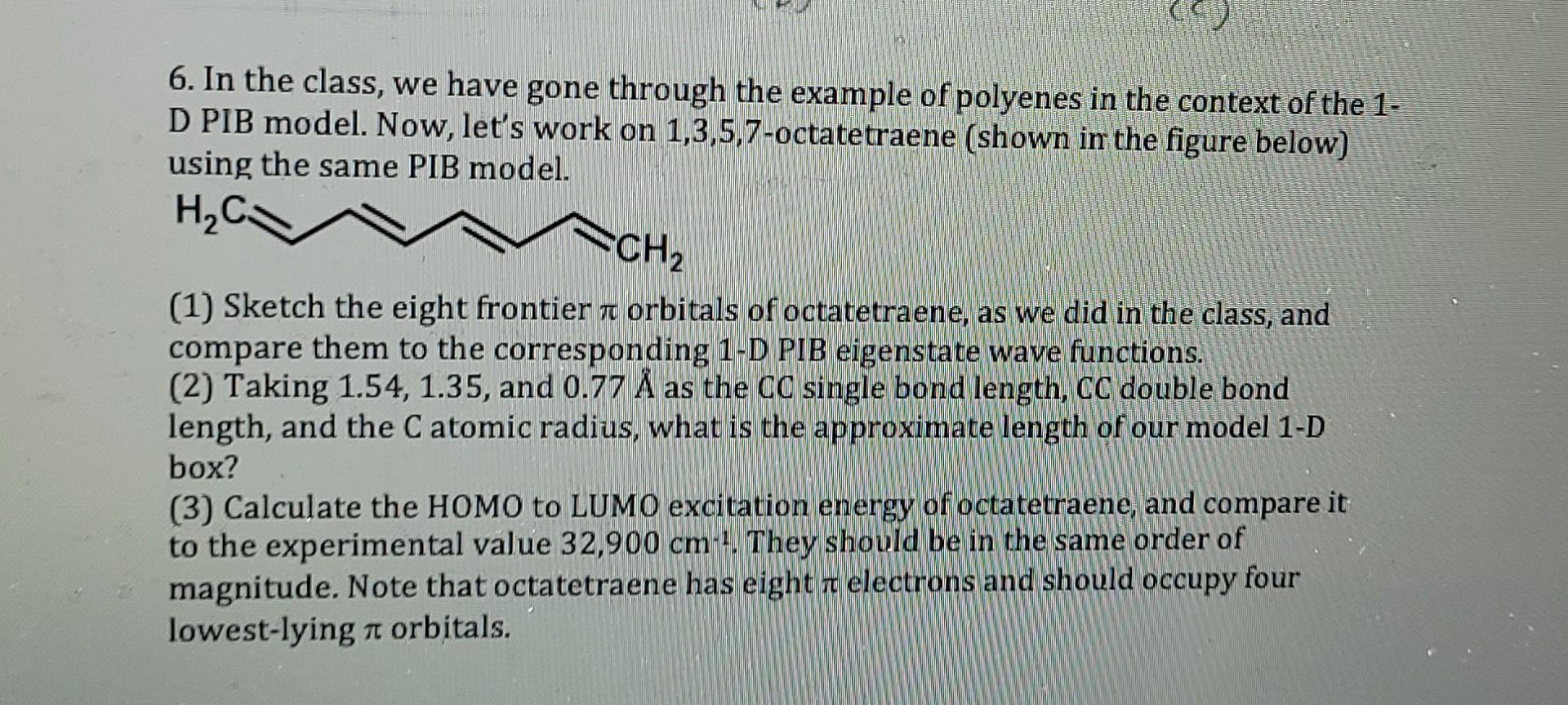
6. In the class, we have gone through the example of polyenes in the context of the 1. D PIB model. Now, let's work on 1,3,5,7-octatetraene (shown in the figure below) using the same PIB model. HC. CH2 TO (1) Sketch the eight frontier a orbitals of octatetraene, as we did in the class, and compare them to the corresponding 1-D PIB eigenstate wave functions. (2) Taking 1.54, 1.35, and 0.77 as the CC single bond length, CC double bond length, and the C atomic radius, what is the approximate length of our model 1-D box? (3) Calculate the HOMO to LUMO excitation energy of octatetraene, and compare it to the experimental value 32,900 cm 1. They should be in the same order of magnitude. Note that octatetraene has eight r electrons and should occupy four lowest-lying n orbitals
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


