Question
6. The following Pourbiax diagram is for the lead metal +10 +0.75 +0.5 +0.25 E(V) 0 -0.25 -0.5 -0.75 -1.0 Pb2+ Pb 1 A.
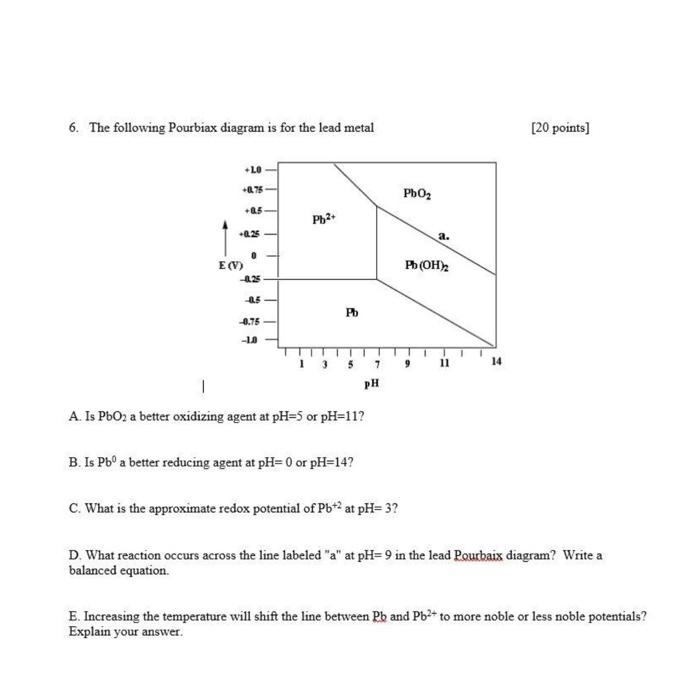
6. The following Pourbiax diagram is for the lead metal +10 +0.75 +0.5 +0.25 E(V) 0 -0.25 -0.5 -0.75 -1.0 Pb2+ Pb 1 A. Is PbO2 a better oxidizing agent at pH-5 or pH=11? B. Is Pb a better reducing agent at pH= 0 or pH=14? 7 PH C. What is the approximate redox potential of Pb+2 at pH= 3? PbO a. Pb(OH)2 9 11 14 [20 points] D. What reaction occurs across the line labeled "a" at pH=9 in the lead Pourbaix diagram? Write a balanced equation. E. Increasing the temperature will shift the line between Pb and Pb+ to more noble or less noble potentials? Explain your answer.
Step by Step Solution
3.29 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1
This is a Pourbaix diagram for lead which shows the equilibrium phases of lead in water at different pH values and electrode potentials E Lets address ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Understanding Basic Statistics
Authors: Charles Henry Brase, Corrinne Pellillo Brase
6th Edition
978-1133525097, 1133525091, 1111827028, 978-1133110316, 1133110312, 978-1111827021
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



