Question: 6.1. (a) Describe and discuss fully the experiments you would conduct and the nature of the results you would hope to obtain if, for a
6.1. (a) Describe and discuss fully the experiments you would conduct and the nature of the results you would hope to obtain if, for a given ceramic oxide of Mg in MgO, you wished to ascertain (Graphic below is to help solve exercise): (1) Whether diffusion rates in a given temperature range occurred by an intrinsic or extrinsic mechanism. (2) Diffusion in a given polycrystalline ceramic was predominantly aong grain boundaries or through the lattice. (3) Whether diffusion occurred via a vacancy mechanism or a ring type of interchange. (b) What concentration of trivalent impurity is required to make the cation diffusivity of Mg++ in MgO extrinsic to its melting point. Explain fully all estimates of property values made in your calculation.

6.2. The application of pressure (not necessarily hydrostatic) has been observed to allect several processes which are presumed to be diffusion-controlled. Give several ways in which pressure can affect self-diffusion coefficients and the expected direction of change in D with increasing pressure for (o) vacancy diffusion and (b) interstitial diffusion. (The equation below is to help solve exercise).
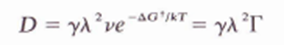
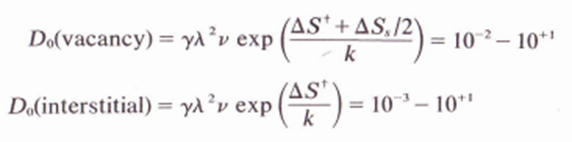
6.3. If diffusion anneal times are doubled, at a given temperature, average penetration depths for the diffusing species will increase by a factor of ____________________.
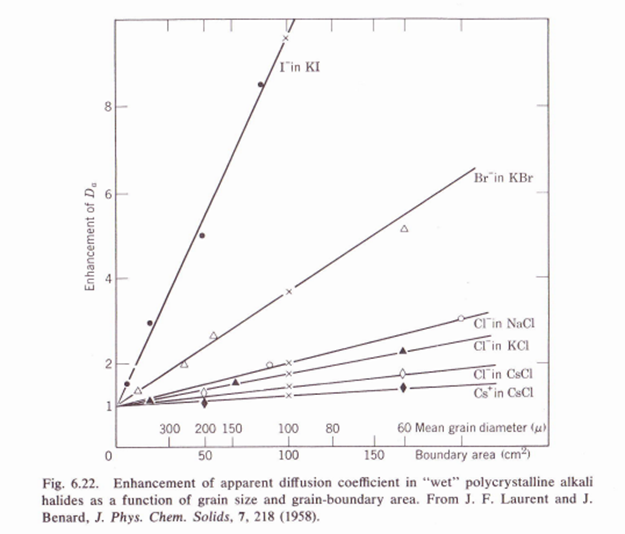
6.4. Discuss the influence of zinc chloride addition (10-4moleV%) to the diffusivity of all ions (Zn, Na, and Cl) in a single-crystal NaCl from room temperature to the melting temperature. (Graphic below is to help solve exercise).
6.5. From the sintering data on ZnS, diffusion coefficients were measured. At 563C, a diffusion coefficient of 3 x 10-4cm2/sec was measured; at 450C 1.0 x 10-4cm2/sec. (a) Determine the activation energy and D0. (b) From your knowledge of the structure, predict the nature of the activation energy from the point of view of movement and creation of defects. (c) On the basis of similarity with ZnO predict the change in D with partial pressure of sulfur.
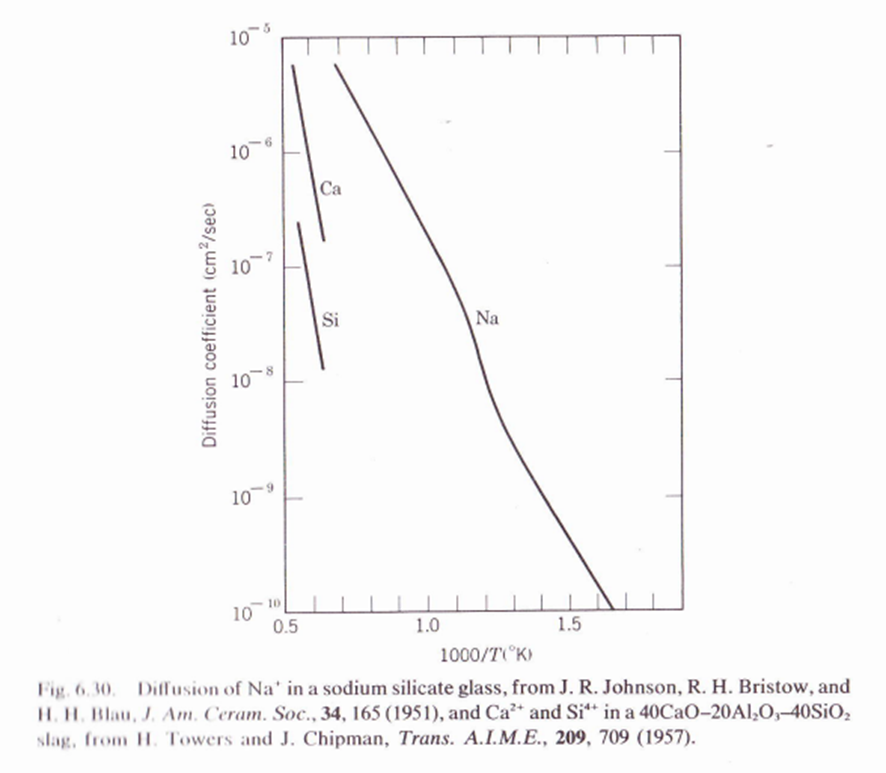
6.6. Figure 6.30 shows diffusion coemcients for ions in an annealed sodium-calcium silicate glass. (a) Why does Na+ diffuse faster than Ca++ and Si+4? (b) What is the nonlinear part of the Na+ diffusion curve due to? (c) How would quenching the glass change the plot? (d) What is the activation energy (experimenta) for Na+ diffusion in the liquid state of the glass. (Graphic below is to help solve exercise).
6.7. (a) What is the predicted oxygen partial pressure dependence of iron ion diffusion in iron deficient Fe3O4? (b) What is the predicted oxygen partial pressure dependence of oxygen diffusion in iron excess Fe3O4?
6.8. A student decides to study Ca diffusion in NaCl. It is known that Ca diffuses via a vacancy mechanism on the Na sublattice and that over the range of experimentation [CaNa]: [VNa]. Show that DCa is a function of [CaNa]; thus c/t D2c/x2. (Graphic below is to help solve exercise).
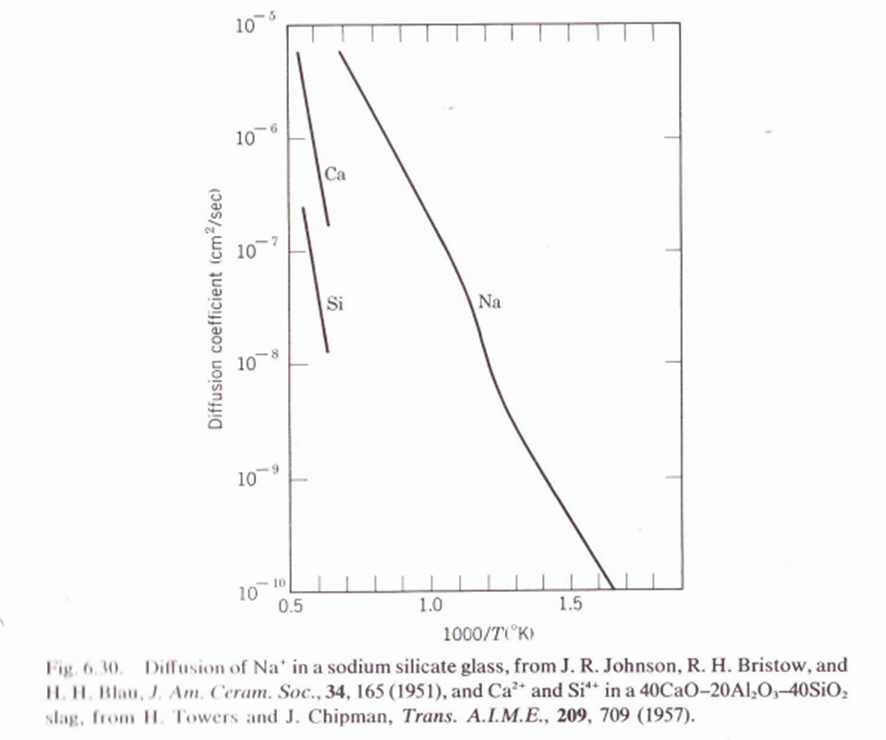
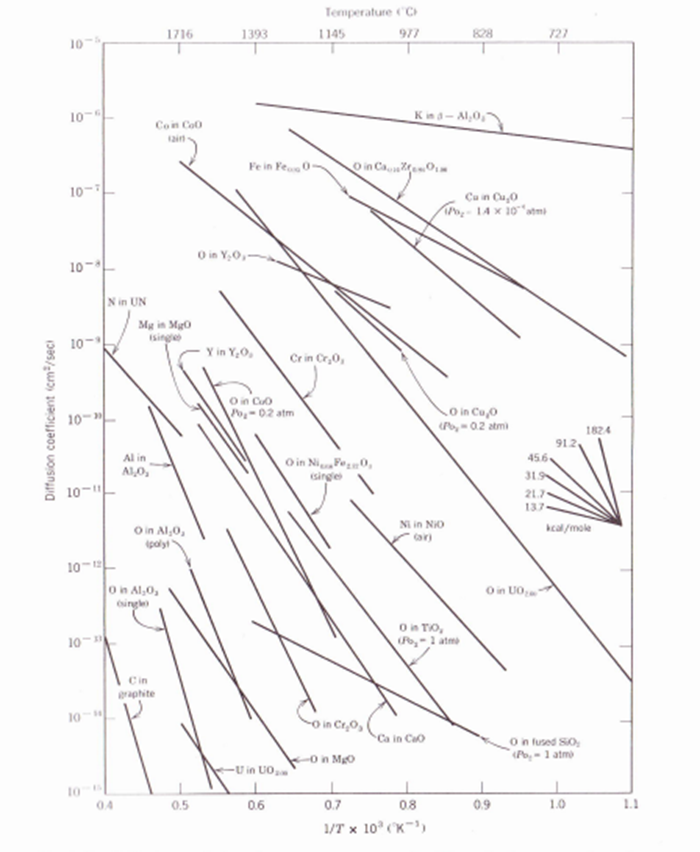
Fig. 6.11. Diffusion coefficients in some common ceramics. The activation energy Q can be estimated from the slope and the insert, for example, for O in Ca0.14Zr0.msO1.ms the Q=29kcal/mole. D=2e/kT=2 D0(vacancy)D0(interstitial)=2exp(kS+Ss/2)=10210+1=2exp(kS)=10310+1 F Benard, J. Phys. Chem. Solids, 7, 218 (1958). Fig. 6.3 and 11. HI. Blau, J. Am. Ceram. Soc., 34, 165 (1951), and Ca2+ and Si4+ in a 40CaO20Al2O340SiO2 slag, from II. Towers and J. Chipman, Trans. A.I.M.E., 209, 709 (1957). Fig. 6. 36 1I. HI. Blau, J. Am. Ceram. Soc., 34, 165 (1951), and Ca2+ and Si4+ in a 40CaO20Al2O340SiO2 slag, from II. Towers and J. Chipman, Trans. A.I.M.E., 209, 709 (1957)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


