Question
6p Determine the number of atoms in a solid square disc of pure Silicon with sides measuring 6.9 cm and a thickness of 1.6
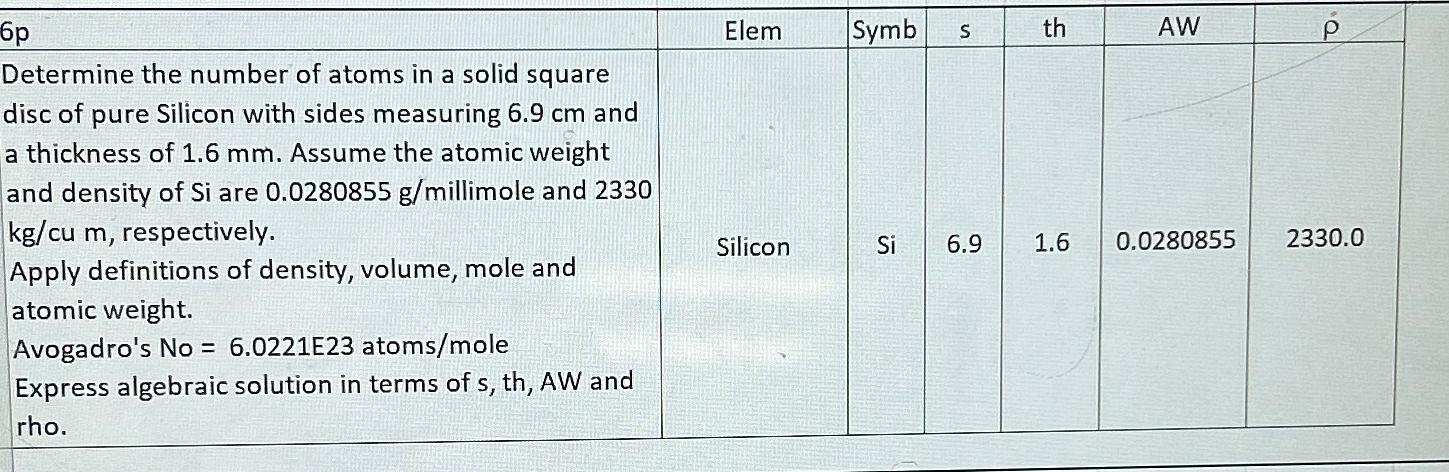
6p Determine the number of atoms in a solid square disc of pure Silicon with sides measuring 6.9 cm and a thickness of 1.6 mm. Assume the atomic weight and density of Si are 0.0280855 g/millimole and 2330 kg/cu m, respectively. Apply definitions of density, volume, mole and atomic weight. Avogadro's No = 6.0221E23 atoms/mole Express algebraic solution in terms of s, th, AW and rho. Elem Silicon Symb S th AW Si 6.9 1.6 0.0280855 2330.0
Step by Step Solution
There are 3 Steps involved in it
Step: 1
The question is asking us to calculate the number of silicon atoms present in a solid square disc with given dimensions Its important to note that wer...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemistry The Central Science
Authors: Theodore Brown, Eugene LeMay, Bruce Bursten, Catherine Murphy, Patrick Woodward
12th edition
321696727, 978-0132175081, 978-0321696724
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



