Answered step by step
Verified Expert Solution
Question
1 Approved Answer
(6pts) Question 2 Consider the equation for the dissolution of calcium in acid, Ca(s) + 2 H+(aq) Ca2+ (aq) + H2(g) (2pts) Calculate the minimum
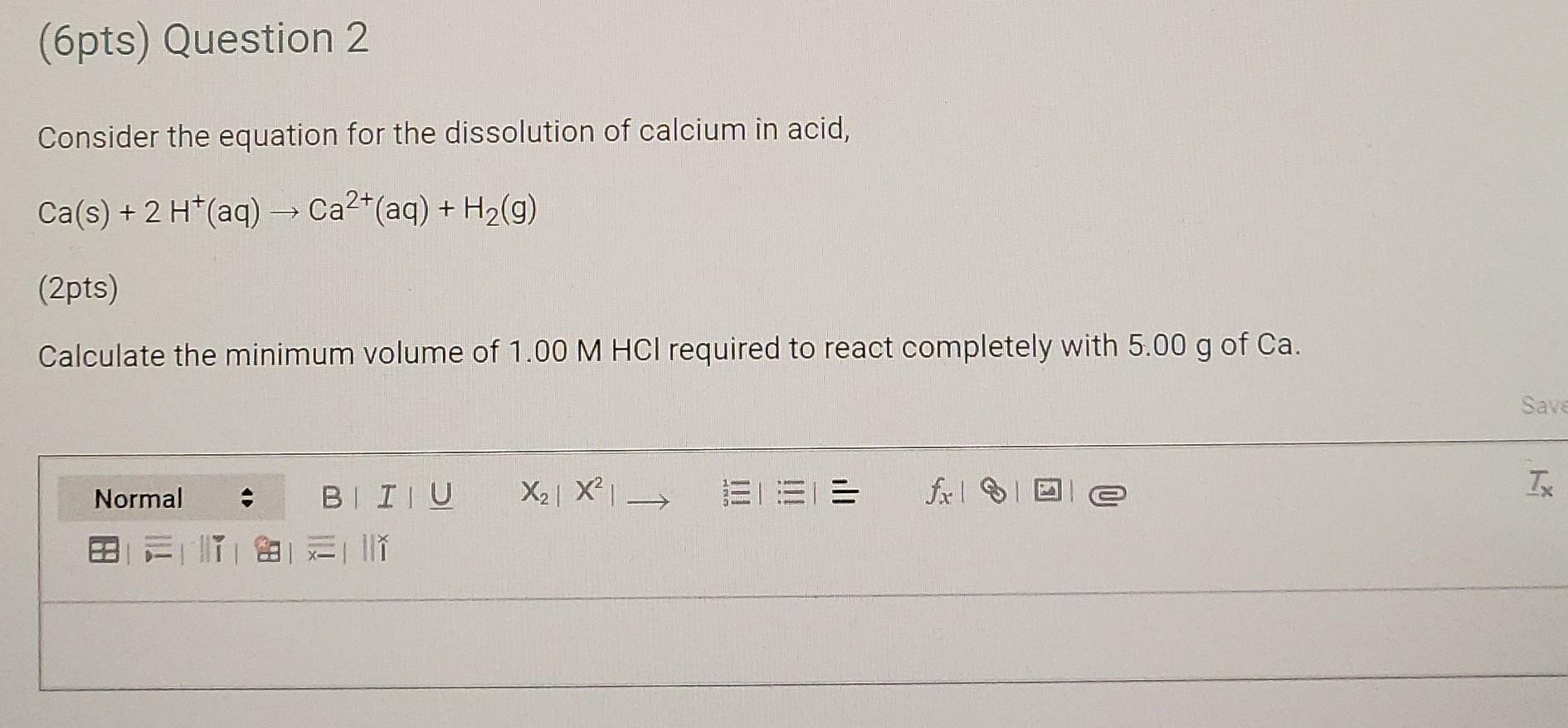
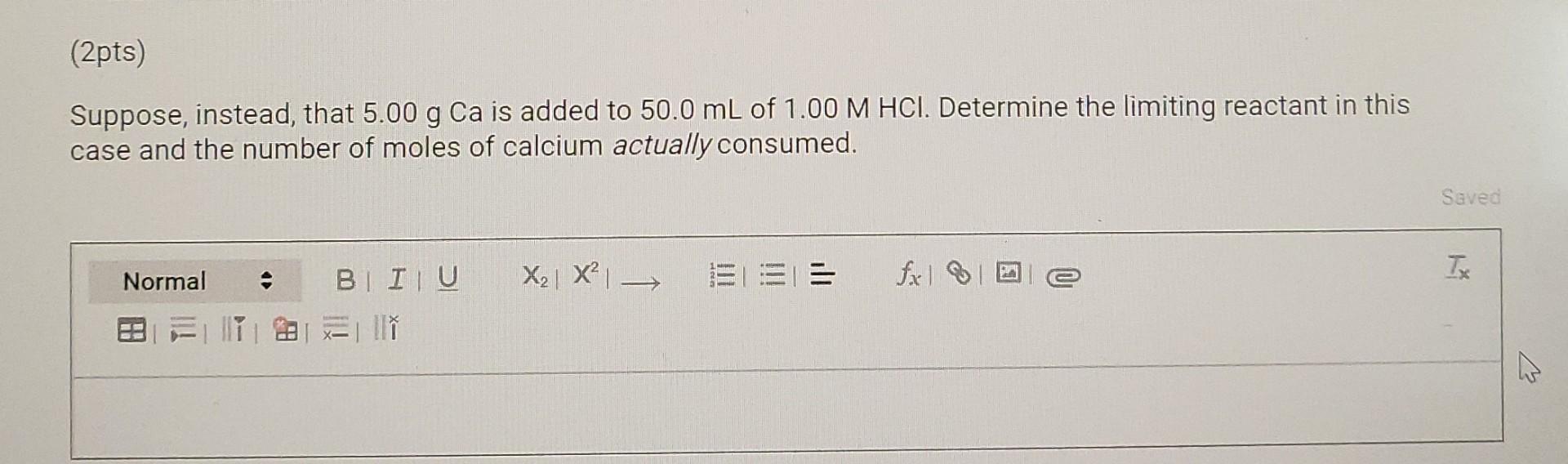
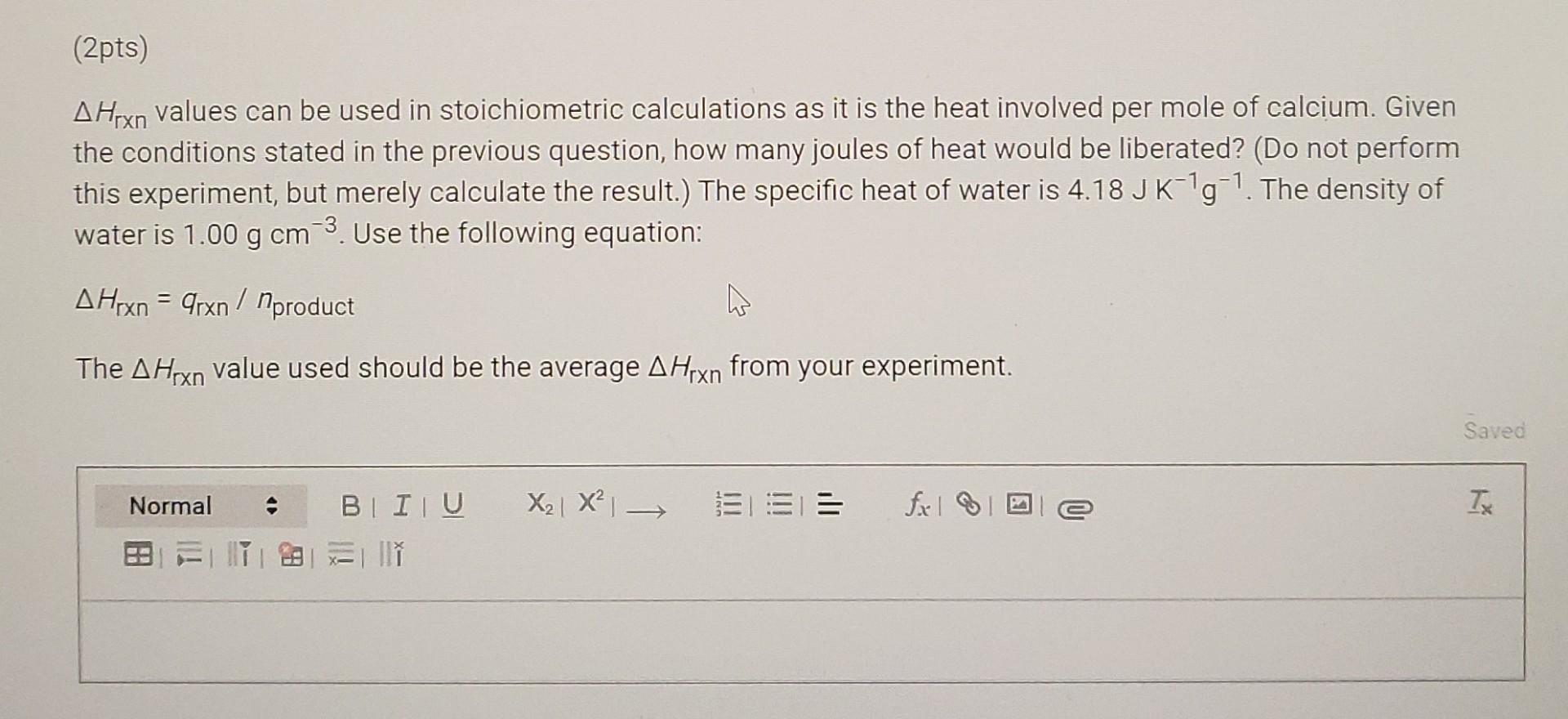
(6pts) Question 2 Consider the equation for the dissolution of calcium in acid, Ca(s) + 2 H+(aq) Ca2+ (aq) + H2(g) (2pts) Calculate the minimum volume of 1.00 M HCl required to react completely with 5.00 g of Ca. Save X2 X EL== fall Tx Normal BI ITU ESTIM (2pts) Suppose, instead, that 5.00 g Ca is added to 50.0 mL of 1.00 M HCl. Determine the limiting reactant in this case and the number of moles of calcium actually consumed. Saved Normal X2| X = = T fx la -> BI IU 2 l w (2pts AHrxn values can be used in stoichiometric calculations as it is the heat involved per mole of calcium. Given the conditions stated in the previous question, how many joules of heat would be liberated? (Do not perform this experiment, but merely calculate the result.) The specific heat of water is 4.18 JK'g-1. The density of water is 1.00 g cm 3. Use the following equation: A Hrxn = 9rxn nproduct The AHrxn value used should be the average A Hrxn from your experiment. Saved Normal == fixel 2 TX BI IU X2 X 28 II (6pts) Question 2 Consider the equation for the dissolution of calcium in acid, Ca(s) + 2 H+(aq) Ca2+ (aq) + H2(g) (2pts) Calculate the minimum volume of 1.00 M HCl required to react completely with 5.00 g of Ca. Save X2 X EL== fall Tx Normal BI ITU ESTIM (2pts) Suppose, instead, that 5.00 g Ca is added to 50.0 mL of 1.00 M HCl. Determine the limiting reactant in this case and the number of moles of calcium actually consumed. Saved Normal X2| X = = T fx la -> BI IU 2 l w (2pts AHrxn values can be used in stoichiometric calculations as it is the heat involved per mole of calcium. Given the conditions stated in the previous question, how many joules of heat would be liberated? (Do not perform this experiment, but merely calculate the result.) The specific heat of water is 4.18 JK'g-1. The density of water is 1.00 g cm 3. Use the following equation: A Hrxn = 9rxn nproduct The AHrxn value used should be the average A Hrxn from your experiment. Saved Normal == fixel 2 TX BI IU X2 X 28
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


