Answered step by step
Verified Expert Solution
Question
1 Approved Answer
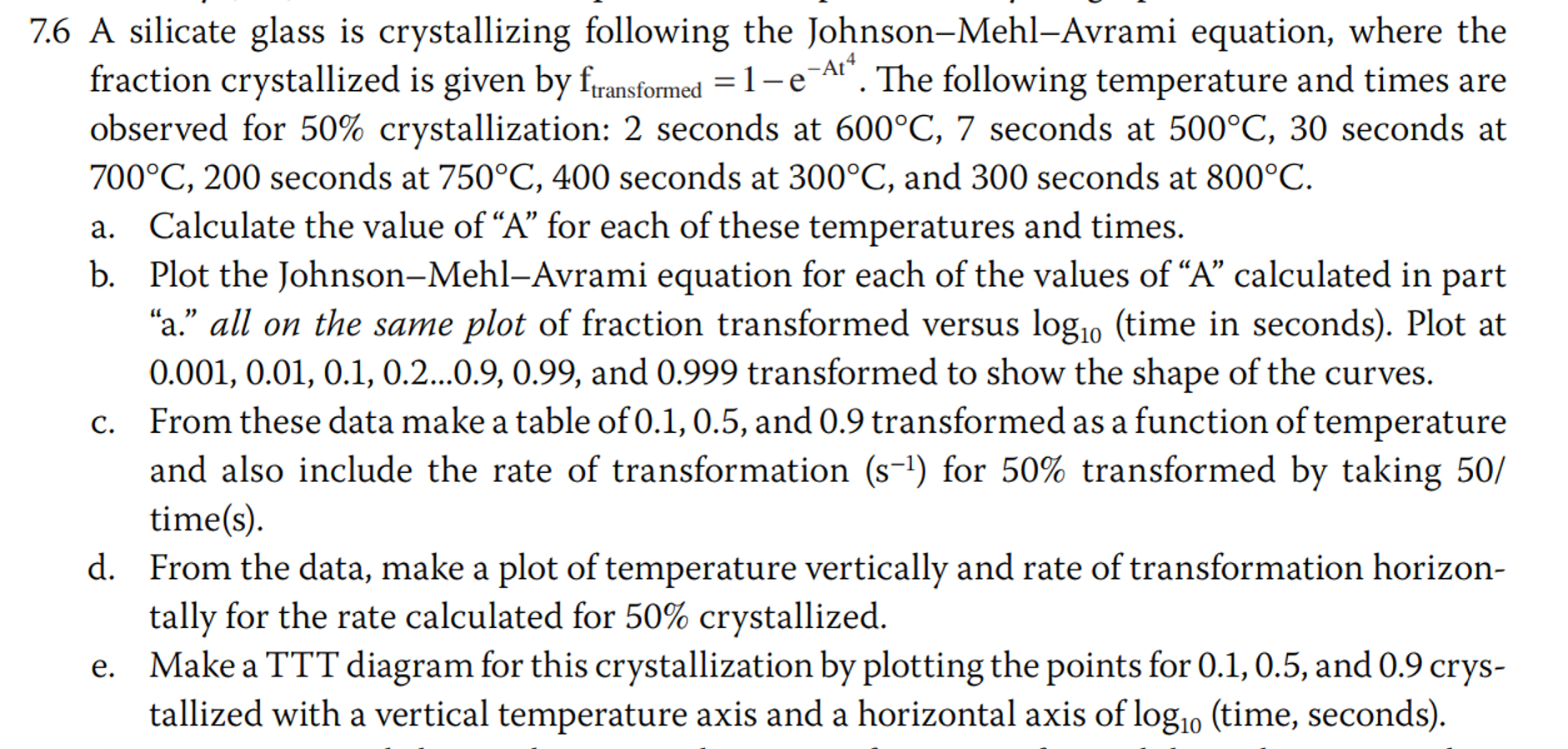
7 . 6 A silicate glass is crystallizing following the Johnson - Mehl - Avrami equation, where the fraction crystallized is given by f t
A silicate glass is crystallizing following the JohnsonMehlAvrami equation, where the fraction crystallized is given by The following temperature and times are observed for crystallization: seconds at seconds at seconds at seconds at seconds at and seconds at
a Calculate the value of A for each of these temperatures and times.
b From these data make a table of and transformed as a function of temperature
and also include the rate of transformation for transformed by taking
times
c From the data, make a plot of temperature vertically and rate of transformation horizon
tally for the rate calculated for crystallized.
d Make a TTT diagram for this crystallization by plotting the points for and crys
tallized with a vertical temperature axis and a horizontal axis of time seconds
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


