Question
7. For the decomposition of (CH3)20 (species A) at 777 K, the time required for [A], to fall to 0.69[A], as a function of
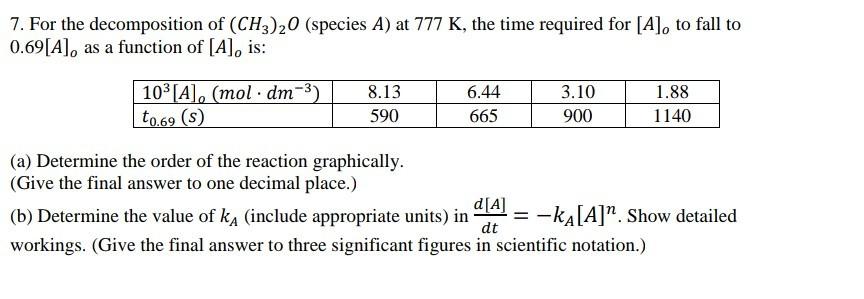
7. For the decomposition of (CH3)20 (species A) at 777 K, the time required for [A], to fall to 0.69[A], as a function of [A], is: 103 [A], (mol dm-3) to.69 (s) 8.13 6.44 3.10 1.88 590 665 900 1140 (a) Determine the order of the reaction graphically. (Give the final answer to one decimal place.) d[A] (b) Determine the value of ka (include appropriate units) in = -kA[A]*. Show detailed dt workings. (Give the final answer to three significant figures in scientific notation.)
Step by Step Solution
3.40 Rating (150 Votes )
There are 3 Steps involved in it
Step: 1
Answer a The order of th...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Physical Chemistry
Authors: Peter Atkins
7th Edition
978-0716735397, 716735393, 978-0716743880
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



