Question
7. In a simple classical model for hydrogen, it can have a dipole moment of about 110-23 Am. Assuming the radius of hydrogen is
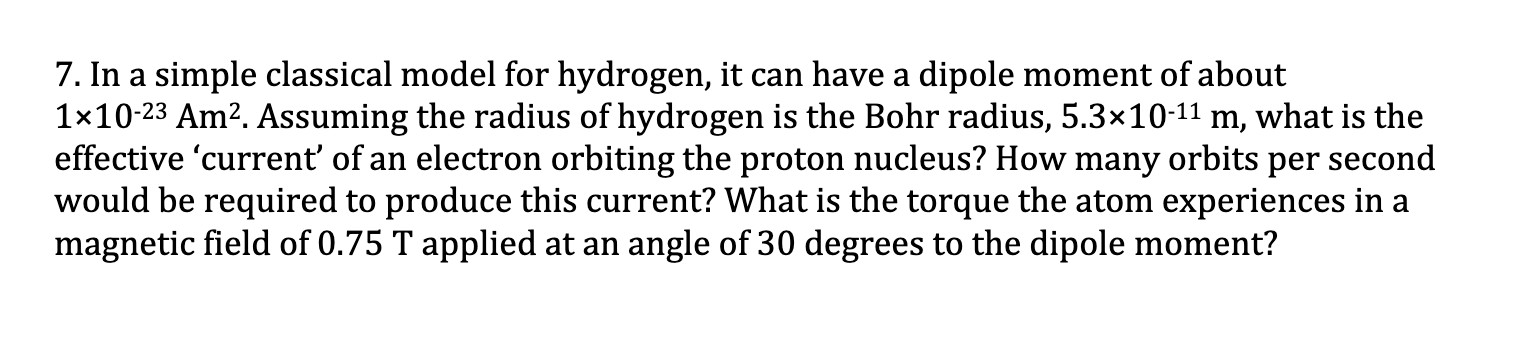
7. In a simple classical model for hydrogen, it can have a dipole moment of about 110-23 Am. Assuming the radius of hydrogen is the Bohr radius, 5.310-11 m, what is the effective 'current' of an electron orbiting the proton nucleus? How many orbits per second would be required to produce this current? What is the torque the atom experiences in a magnetic field of 0.75 T applied at an angle of 30 degrees to the dipole moment?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Particle Physics
Authors: Brian R. Martin, Graham Shaw
4th Edition
1118912164, 1118912160, 978-1-118-9119, 978-1118912164
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



