Answered step by step
Verified Expert Solution
Question
1 Approved Answer
7 Label each of the phases in these two diagram. Apply the Classius-Clayperon equation to interpret the given figure. By using thermodynamics principle why the
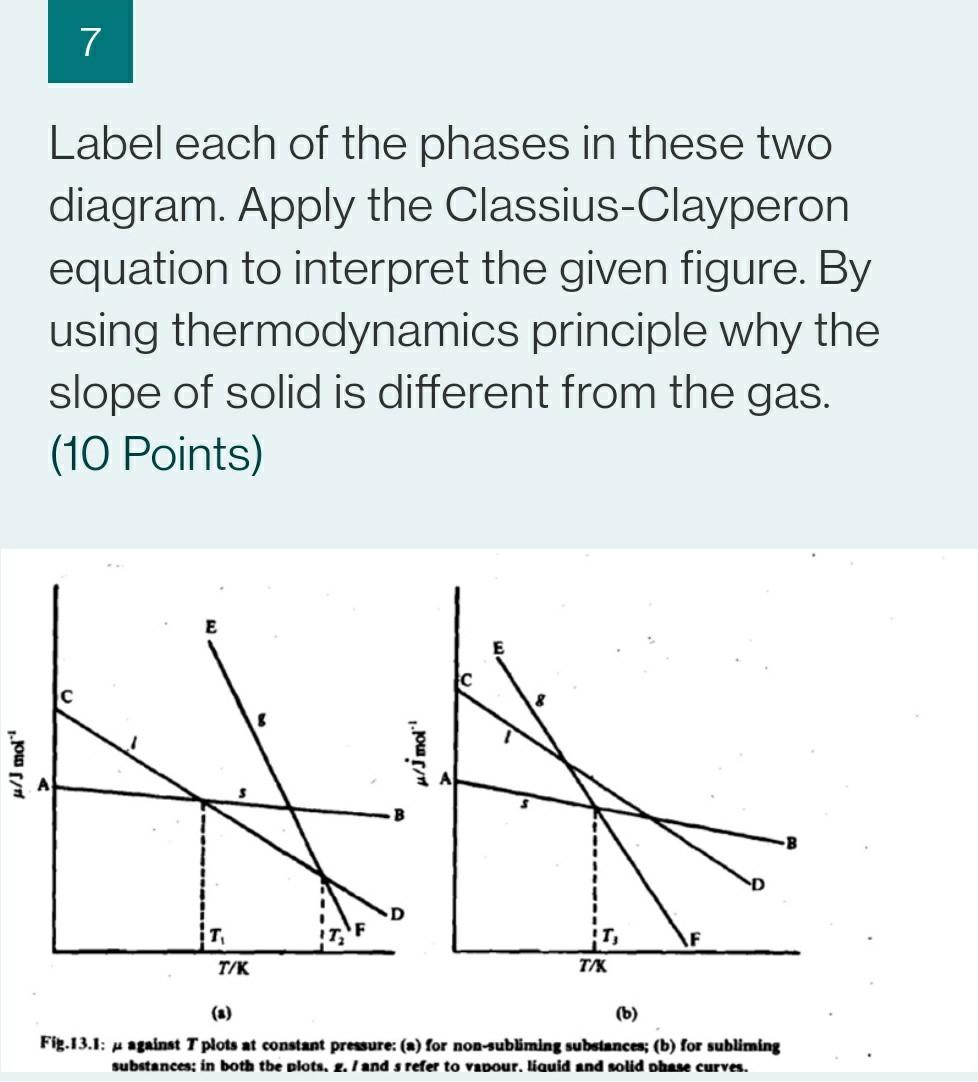
7 Label each of the phases in these two diagram. Apply the Classius-Clayperon equation to interpret the given figure. By using thermodynamics principle why the slope of solid is different from the gas. (10 Points) E $ w/mol Mlj mol T T/K TAK (b) Fig.13.1: - against T plots at constant pressure: (a) for non-subliming substances; (b) for subliming substances; in both the plots. 2. / and s refer to vapour.liquid and solid phase curves
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


