Question
7. On the 2-component phase diagram shown below at 1 atm pressure, apply the phase rule to points X, Y and Z. a. How
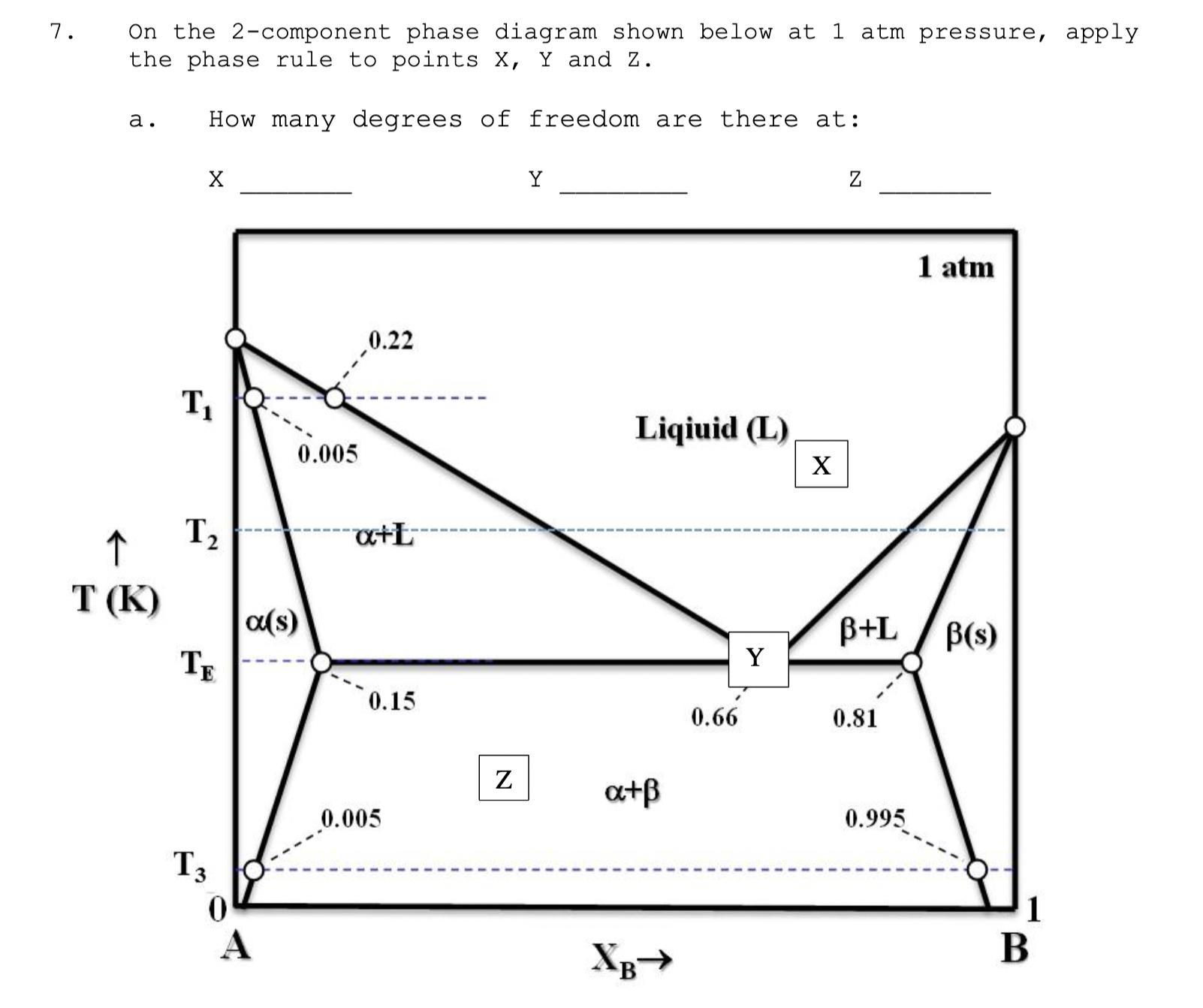
7. On the 2-component phase diagram shown below at 1 atm pressure, apply the phase rule to points X, Y and Z. a. How many degrees of freedom are there at: Y Z T 0.005 0.22 T (K) 12 TE (s) +L 0.15 Is 0 A 0.005 N Liqiuid (L) a+B 0.66 XB 1 atm B+L (s) Y 0.81 0.995 1 B
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Statistics For Engineers And Scientists
Authors: William Navidi
3rd Edition
73376345, 978-0077417581, 77417585, 73376337, 978-0073376332
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



