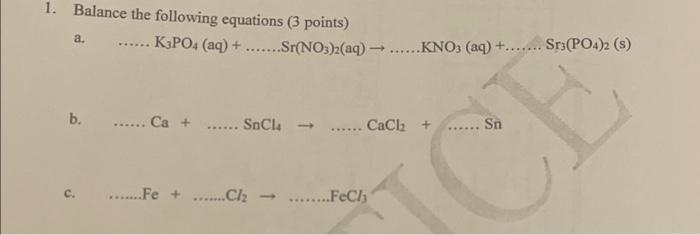7. What is the correct chemical name for both Na2SO4 and N2S ? a. Sodium sulfate and sodium sulfide b. Sodium disulfate and sodium sulfate c. Sodium sulfide and sodium sulfide d. Sodium disulfide and sodium sulfite 8. A compound of oxygen and carbon contains 57.2% oxygen and 47.8% carbon. What is its: empirical fomtula? 14. CO b. CO2 c. CO3 d. C2O3 9. The compound in 8 above has a molar mass of 288g, what is its molecular formula? a. CO b. CO2 c. CO3 d. C2O3 10. How many atoms are there in 42g of silicon (Si)? a. 1.511020 b. 3.011023 c. 6.02102 d. 9.031023 11. How many moles of chromium is 17 grams of chromium? a. 0.33 b. 3.06 c. 884 d. 1.971023 12. What is the percentage of sulphur in the compound SO2 ? a. 20 b. 25 c. 50 d. 75 13. Identify the type of redox reaction taking place in the equation below: 2NaI+Br2NaBr+I2 a. Disproportionation b. Metal displacement c. Decomposition d. Halogen displacement Depariment of Math and Science 14. What is the cocrect name for the compound with moleculat formula MaOS2 a. Manganese dioxide b. Manganese (ii) oxide c. Manganese (IV) oxide d. Diminganese exide 15. What is the oxidation number for phosphorous in the PF6 " ion? a. 1 b. 5 c. +5 d. +1 16. For the calculation 6.022103 divided by 12.011=501.3737407, what is the correct number of significant figures for the answer? a. 1 b. 2 c. 3 d. 4 17. How many moles of MggCl2 are present in 60.0mL of 0.100MMgCl solution? a. 0.0006 b. 0.006 c. 0.06 d. 0.6 SECTION B - Short Answers and Caleulations [Total 30 marks] Inswer all questions in the space provided, showing all calcwlations and steps clearly. Marks for each westion are indicated. 1. i) Are the following compounds likely to be ionic or molecular? a) Li2S. b) CF4 [2] ii) Give the number of electrons in each of the following common ions: a) Li+..k.... b) S2 [2] iii) Write the formula for the following compounds: a) sulphur trioxide b) tin (IV) chloride [2] What is its empirical formula? 3. The empirical formula of a compound is OH. If the molar mass of the compound is about 51g, what is its molecular formula? [3] 4. How many sodium atoms are there in 30.7g of N? [2] 5. MnO2+4HClMnCl2+Cl2+2H2O i) Based on the equation above, if 14.9g of MnO2 and 17.2g of HCl react, which reagent is the limiting reagent? ii) How many grams of ehlorine (Cl) will be produced? iii) If 4.38g of chlorine were produced in the experiment, what is the percentage yield of the reaction? [1] 6. Calculate the molarity of the following solution: a. 13.9g of methanol (CH3OH) in 497mL of solution [2] 7. Write ionic and net ionic equations for the following: a. Na2S(aq)+ZnCl2 (aq) 2NaCl(aq)+ZnS (s) [2] 1. Balance the following equations ( 3 points) a. ...K3PO4(aq)+...Sr(NO3)2(aq)..KNO3(aq)+..Sr3(PO4)2(s) b. .Ca+.SnCl4.CaCl2+..Sn c. Fe+..Cl2 FeCl3